Rink Amide Resin |
| Katalog-Nr.GA11149 |
Products are for research use only. Not for human use. We do not sell to patients.
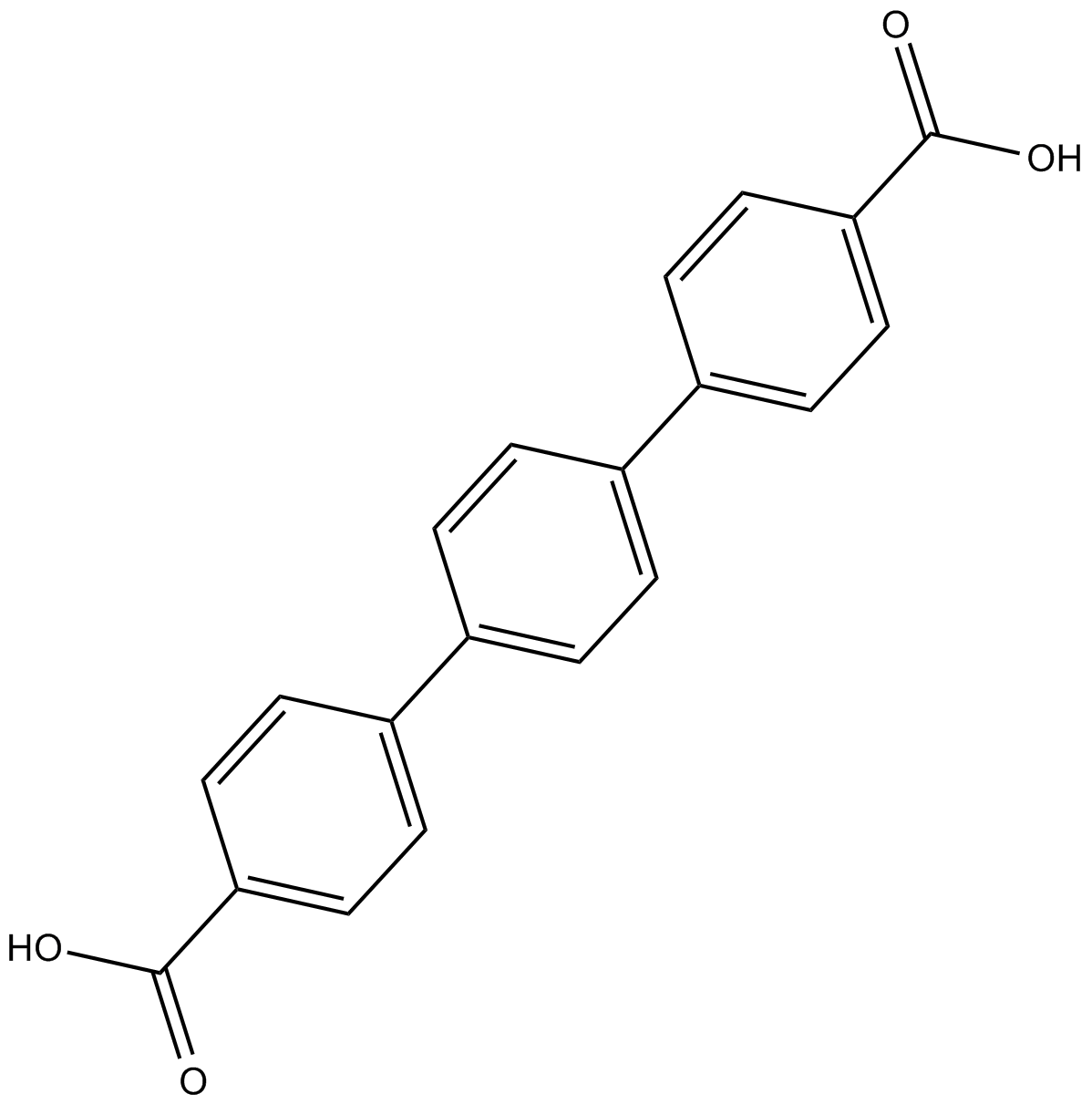
Cas No.: 13653-84-4
Sample solution is provided at 25 µL, 10mM.
Rink resin is originally developed for SPS of peptide amides. Now the scope of its application is extended from carboxylic amides to the immobilization of amines, substituted amides. Libaries of primary amines have been synthesized by the treatment of Rink amine resin with aldehyde to form aldimines, which are subsequently reacted with Grignard reagents or lithium reagents to yield amines that are not commercially available. These amines are released from resin by treatmnet with TFA-water-DCM (5:5:90) for 5 h at room temperature. N-Substituted amides are obtained by reducing the above-mentioned aldimines with Na(CN)BH3 to the corresponding amines, followed by acylation with acid chlorides or symmetrical anhydrides. The produts are cleaved with TFA-water-DCM (5:1:94) for 20 min at room temperature. Direct functionalization of Rink amide resin with nucleophiles has also been reported. The Fmoc protecting group can be readly removed with 20% piperidine in DMF prior to the above manipulation.
Rink amide resin: 4-(2',4'-Dimethoxyphenyl-Fmoc-aminomethyl-phenoxy-acetamido- norleucylaminomethyl resin; Substitution: 0.4 - 0.8 mmole/g resin; Bead size 100- 200 mesh (polystyrene- 1% DVB)
Structure: Application: Li et al reported a simple, clean, high yielding and linker-free method for the synthesis of disubstituted guanidines by using Rink amide resin as an amine component [1]. Decomposition of the resin linkers during TFA cleavage of the peptides in the Fmoc strategy leads to alkylation of sensitive amino acids. The C-terminal amide alkylation, reported for the first time, is shown to be a major problem in peptide amides synthesized on the Rink amide resin. This side reaction occurs as a result of the Rink amide linker decomposition under TFA treatment of the peptide resin. The use of 1,3-dimethoxybenzene in a cleavage cocktail prevents almost quantitatively formation of C-terminal N-alkylated peptide amides. Oxidized by-product in the tested Cys- and Met-containing peptides were not observed, even if thiols were not used in the cleavage mixture[2].
Reference:
[1] Min Li, Lawrence J. Wilson and David E. Portlock. A simple solid-phase synthesis of disubstituted guanidines using Rink amide resin as an amine component. Tetrahedron Letters 42 (2001) 2273–2275
[2] Stathopoulos P, Papas S, Tsikaris V. C-terminal N-alkylated peptide amides resulting from the linker decomposition of the Rink amide resin: a new cleavage mixture prevents their formation. J Pept Sci. 2006;12(3):227-32.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















