BTSA1 |
| Catalog No.GC39355 |
BTSA1 est un activateur BAX puissant, de haute affinité et actif par voie orale avec un IC50 de 250 nM et un EC50 de 144 nM. BTSA1 se lie avec une affinité et une spécificité élevées au site d'activation N-terminal et induit des changements conformationnels À BAX conduisant À l'apoptose médiée par BAX.
Products are for research use only. Not for human use. We do not sell to patients.
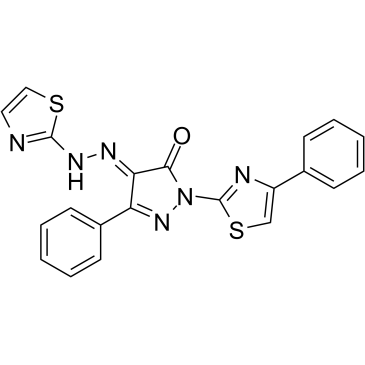
Cas No.: 314761-14-3
Sample solution is provided at 25 µL, 10mM.
BTSA1 is a potent, high affinity and orally active BAX activator with an IC50 of 250 nM and an EC50 of 144 nM. BTSA1 binds with high affinity and specificity to the N-terminal activation site and induces conformational changes to BAX leading to BAX-mediated apoptosis[1].
[1]. Reyna DE, et al. Direct Activation of BAX by BTSA1 Overcomes Apoptosis Resistance in Acute Myeloid Leukemia. Cancer Cell. 2017 Oct 9;32(4):490-505.e10.
Average Rating: 5 (Based on Reviews and 32 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















