Fexofenadine HCl (Synonyms: MDL 16455A) |
| Catalog No.GC10966 |
Le chlorhydrate de fexofénadine (MDL-16455) est un antagoniste des récepteurs H1 actif par voie orale et non sédatif.
Products are for research use only. Not for human use. We do not sell to patients.
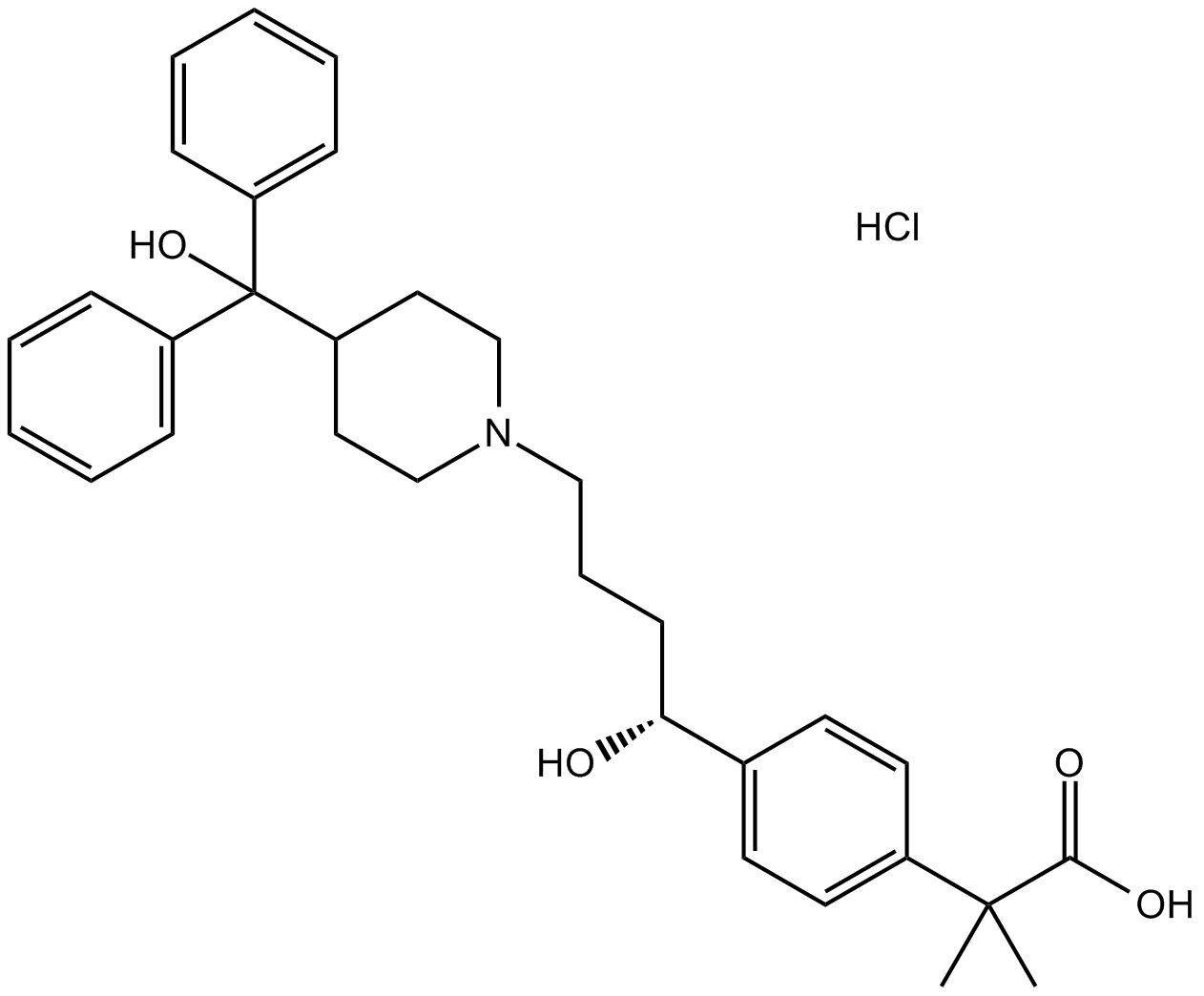
Cas No.: 153439-40-8
Sample solution is provided at 25 µL, 10mM.
Fexofenadine hydrochloride (MDL-16455 hydrochloride), a H1R antagonist, is an anti-allergic agent used in seasonal allergic rhinitis and chronic idiopathic urticarial (person aged ≥16 years)[1].
Fexofenadine hydrochloride (MDL-16455 hydrochloride) (100 µM; 1 hour) effectively blocks phosphorylated p38 activation in histamine-induced nasal fibroblasts[2].
Fexofenadine hydrochloride (MDL-16455 hydrochloride) (5-20 mg/kg; oral daily for 3 weeks) dose-dependently suppresses eosinophilia in C57BL/6 mice infected with T. spiralis[1].
References:
[1]. Watanabe N, et al. The effects of fexofenadine on eosinophilia and systemic anaphylaxis in mice infected with Trichinella spiralis. Int Immunopharmacol. 2004 Mar;4(3):367-75.
[2]. Park IH, et al. Histamine Promotes the Release of Interleukin-6 via the H1R/p38 and NF-κB Pathways in NasalFibroblasts. Allergy Asthma Immunol Res. 2014 Nov;6(6):567-72.
Average Rating: 5 (Based on Reviews and 17 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















