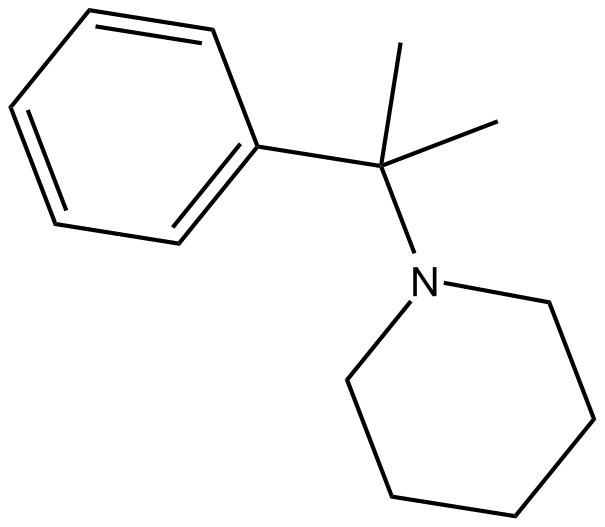
2-Phenyl-2-(1-piperidinyl)propane (Synonyms: 1-(α,α-dimethylbenzyl)-Piperidine,PPP) |
| カタログ番号GC12262 |
2-フェニル-2-(1-ピペリジニル)プロパンは、5.1 μM の IC50 と 5.6 の Ki を持つ選択的かつ可逆的なヒト CYP2B6 阻害剤です。
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 92321-29-4
Sample solution is provided at 25 µL, 10mM.
KI: 11 microM for 7-(benzyloxy)resorufin O-dealkylation activity of liver microsomes obtained from phenobarbital-induced rats
2-Phenyl-2-(1-piperidinyl)propane is a mechanism-based inactivator of human cytochrome P450 (CYP) 2B6.
The use of selective chemical inhibitors of human cytochrome P450 enzymes is a powerful method by which the relative contributions of different human P450 enzymes to the drug metabolism can be obtained. However, the contribution of CYP2B6 in the metabolism is more challenging due to the lack of a well-established inhibitor.
In vitro: Previous study found that 2-phenyl-2-(1-piperidinyl)propane could inactivate the 7-(benzyloxy)resorufin O-dealkylation activity of liver microsomes obtained from phenobarbital-induced rats. The 7-ethoxy-4-(trifluoromethyl)coumarin O-deethylation activity of purified rat liver P450 2B1 and expressed human P450 2B6 was also inactivated by 2-phenyl-2-(1-piperidinyl)propane in a reconstituted system. With NADPH, the loss of activity was founf to be both time- and concentration-dependent, and followed pseudo first order kinetics. The time for 50% of the P450 2B1 to become inactivated at saturating concentrations of 2-phenyl-2-(1-piperidinyl)propane was ~2.5 min. P450 2B6 was inactivated by 2-phenyl-2-(1-piperidinyl)propane with a k(inact) of 0.07 min(-1), a K(I) of 1.2 microM, and a t(1/2) of 9.5 min. The inactivated P450s 2B1 and 2B6 lost about 25 and 15%, respectively, indicating that the loss of activity was caused by a 2-phenyl-2-(1-piperidinyl)propane modification of the apoprotein rather than the heme [1].
In vivo: Up to now, there is no animal in vivo data reported.
Clinical trial: So far, no clinical study has been conducted.
Reference:
[1] Chun J, Kent UM, Moss RM, Sayre LM, Hollenberg PF. Mechanism-based inactivation of cytochromes P450 2B1 and P450 2B6 by 2-phenyl-2-(1-piperidinyl)propane. Drug Metab Dispos. 2000 Aug;28(8):905-11.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















