PPACK
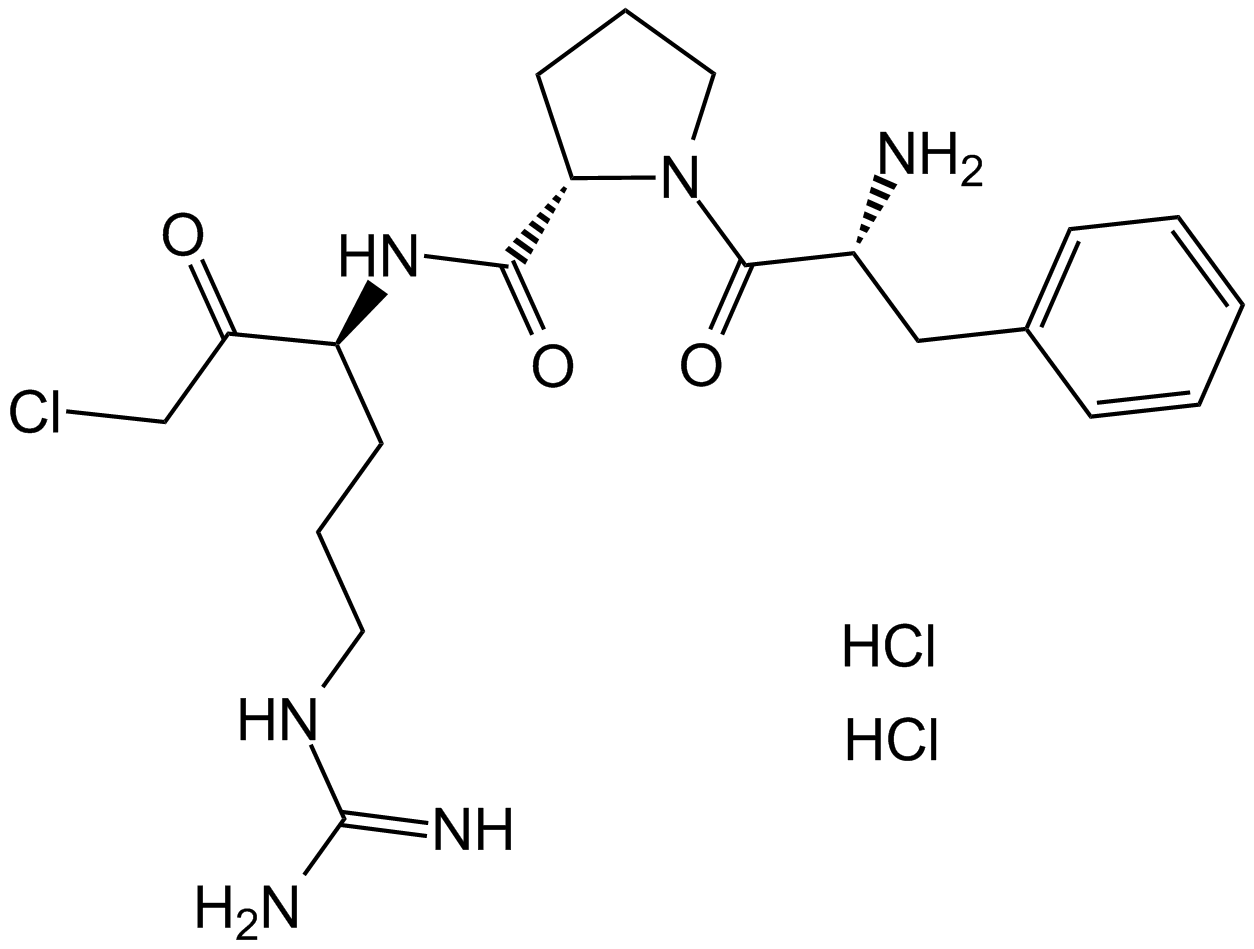
For blood gas and whole-blood electrolyte investigations, D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK), a selective thrombin inhibitor, was assessed as a potential substitute anticoagulant for lithium heparin (LiHep). The synthetic chemical Ppack has a clearly defined structure shown in figure 1. The amino acid sequence and the existence of a chloromethylketone group are indicated by its chemical nomenclature. The molecule's unique amino acid configuration plays a role in its inhibitory effects, especially when it comes to serine proteases. D-phenylalanyl-L-prolyl-L-arginyl is an example of a targeted amino acid sequence that is intended to interact with serine proteases' active site. The protease's active site residues form a covalent link with the chloromethylketone group, which intensifies the inhibitory actions of the enzyme.
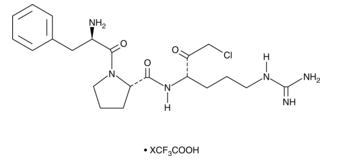
Figure 1: Molecular structure of D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK)
To investigate the effectiveness and safety of using nanoparticles to focally inhibit serine proteases, specifically thrombin (Figure 2), in atherosclerosis. The powerful thrombin inhibitor d-phenylalanyl-l-prolyl-l-arginyl chloromethylketone (PPACK), which has been shown to be effective in preventing the formation of acute thrombi in mouse models of carotid artery thrombosis with minimal bleeding side effects, was carried by the test agent used in this study, a PFC nanoparticle. Moreover, PPACK itself exhibits several orders of magnitude higher affinity for thrombin than other serine proteases, making it a highly effective thrombin inhibitor. We intend to define the role of focal thrombin inhibition in mediating inflammatory signaling events in vitro and in vivo, and how that might play a mechanistic role in reducing vascular endothelial barrier damage and thrombotic risk by administering doses of antithrombin nanoparticles over a period of several weeks. The observed effectiveness of the method implicates thrombin signaling as a major contributor to endothelial damage in this model and shows a considerable direct contribution of thrombin signaling to the genesis of atherosclerosis and the formation of thrombotic risk.
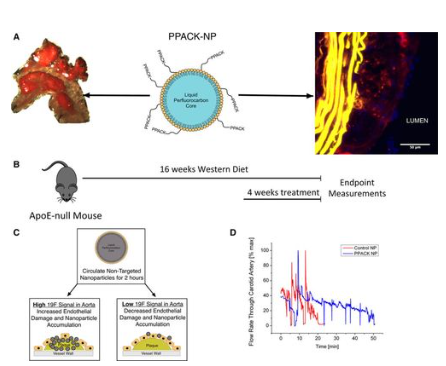
Figure 2: Schematic of d-phenylalanyl-l-prolyl-l-arginyl chloromethylketone (PPACK) nanoparticle (NP) (center panel). Nanoparticles consist of a perfluorocarbon core surrounded by a stabilizing phospholipid monolayer to which PPACK is covalently conjugated.
Groups of ApoE-null mice were given a Western diet for three months, and then the diet was continued for an additional month while receiving treatment (saline, control nanoparticle, and PPACK nanoparticle) to evaluate the effects of the nanoparticle on lowering procoagulant activity. Once the treatment/feeding period was over, the mice were put through a previously proven model) that quantifies the risk of thrombosis by means of photochemical damage to the carotid artery. This model produces a quantitative measure of coagulant activity (vessel occlusion time) that exhibits a good dynamic range and monotonic responsiveness to clotting-affecting therapeutic agents. To allow washout of any remaining antithrombin nanoparticles, animals were allowed to remain on their diets for a further two to three days prior to executing the vascular injury. Following a month of PPACK nanoparticle treatment, carotid occlusion times rose dramatically in comparison to control groups (Figure 3). Occlusion times reached 48.71±6.7 minutes (N=7), compared to 26.11±4.63 minutes (N=9; P=0.005) for saline treatment and 25.55±4.17 minutes (N=9; P=0.004) for control nanoparticle treatment. Despite maintaining a high-fat diet, the occlusion times in the 1-month PPACK nanoparticle-treated group approximated those of previously reported fat-fed ApoE null mice after two months off diet. This suggests that PPACK nanoparticle therapy may more quickly attenuate vessel procoagulant activity in this model than does dietary management.
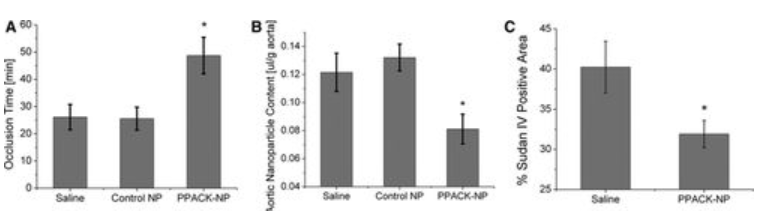
Figure 3: Antithrombin Nanoparticles Reduce Vascular Procoagulant Activity In Vivo.
We examined PPACK nanoparticle treatment's capacity to repair endothelial barriers, which may also lower plaque procoagulant activity as previously documented. For the purpose of magnetic resonance spectroscopy, a dosage of crown ether nanoparticles was injected into 11 mice. The mice were then given two hours to circulate before being killed, at which point plaque saturation takes place. The aorta was removed in its entirety, from the aortic root to the bifurcation, for ex vivo 19F magnetic resonance spectroscopic observations at 11.7T after this circulation period. Figure 3 shows how treating plaque endothelial permeability with PPACK nanoparticles for one month reduced the amount of crown ether nanoparticles deposited (0.084±0.009 μL/g aorta, N=7) in comparison to saline (0.122±0.011 μL/g aorta, N=8; P=0.023) and control nanoparticles (0.132±0.013 μL/g aorta, N=10; P=0.014). We found that there was an inverse connection (R=−0.56; Figure I in the online-only Data Supplement) between plaque permeability and vessel procoagulant activity (P=0.004) using paired samples. This finding is in keeping with our earlier finding that plaque permeability to PFC nanoparticle is correlated with thrombotic risk. For the purpose of measuring the amount of plaque in the aortic arch using computer-assisted planimetry and traditional Sudan IV staining, a subset of mice was selected.16 Overall, aortic arch plaque extent decreased by 20.69% as a result of PPACK nanoparticle treatment (Figure 2): 40.24±3.21% plaque area for mice treated with saline (N=5) and 31.91±1.69% plaque area for mice treated with PPACK nanoparticles (N=7; P=0.03).
Through cell culture investigations, the responses of activated endothelium and monocytic cell lines to thrombin inhibition were measured in order to identify the molecular signaling events that underlie the positive effects of PPACK nanoparticle. Firstly, since the cleavage of the PAR-1 receptor on cell surfaces is the primary mechanism by which thrombin affects different cell types, flow cytometry was employed to ascertain the proportion of intact PAR-1 receptors remaining in each treatment group following thrombin treatment. When compared to thrombin or thrombin/control nanoparticle groups, PPACK nanoparticle treatment completely prevented thrombin cleavage of PAR-1 receptors on human aortic endothelial cells (HAECs). This resulted in significantly lower PAR-1 expression (N=3 for both groups; P=0.019 and P=0.000005, respectively) when compared with baseline.
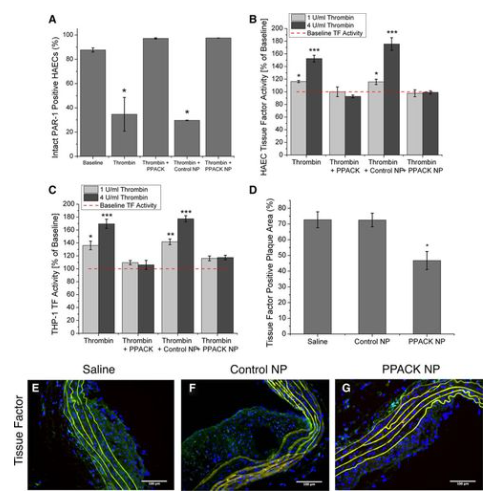
Figure 4: Flow cytometry for intact protease-activated receptor (PAR)-1 after thrombin stimulation in the presence of each treatment group demonstrates inhibition of thrombin-mediated cleavage of PAR-1 with d-phenylalanyl-l-prolyl-l-arginyl chloromethylketone (PPACK) nanoparticle (NP) treatment
We investigated the downstream signaling implications of PAR-1 activation associated to inflammation and coagulation since PPACK nanoparticles effectively reduced PAR-1 activation. A functional assay assessing Factor Xa formation due to tissue factor/factor VIIa (TF/FVIIa) complexes was used to measure tissue factor expression on the surface of HAECs and THP-1 monocytes in response to thrombin stimulation. At both of the thrombin concentrations employed (1 and 4 U/mL), PPACK nanoparticles blocked thrombin-induced TF expression on the surface of both HAECs (Figure 4) and THP-1 monocytes (Figure 4), with no discernible increase above baseline TF levels. As measured by immunofluorescent staining using ImageJ (Figure 4), the entire excised aortic arch segments showed a significant reduction in TF-positive plaque area after PPACK nanoparticle treatment: 46.75±5.74% (N=3) in PPACK-treated mice (Figure 4) versus 72.69±5.06% (N=3; P=0.027) for saline treatment and 72.52±4.34% (N=3; P=0.023) for control nanoparticle treatment.
Furthermore, it's crucial to remember that PPACK, a specific thrombin inhibitor, may not be totally specific to thrombin as a serine protease inhibitor; it can also inhibit other trypsin-like proteases, like Factor Xa. The inhibitory impact of PPACK on Xa has been demonstrated to be three orders of magnitude less than that of thrombin, despite its effects on other proteases. Nevertheless, PPACK continues to be a potent inhibitor of thrombin. However, it's possible that local inhibition of additional coagulation proteases will work in concert to prevent thrombin from activating, albeit in a limited way.













コメント