Yoda1
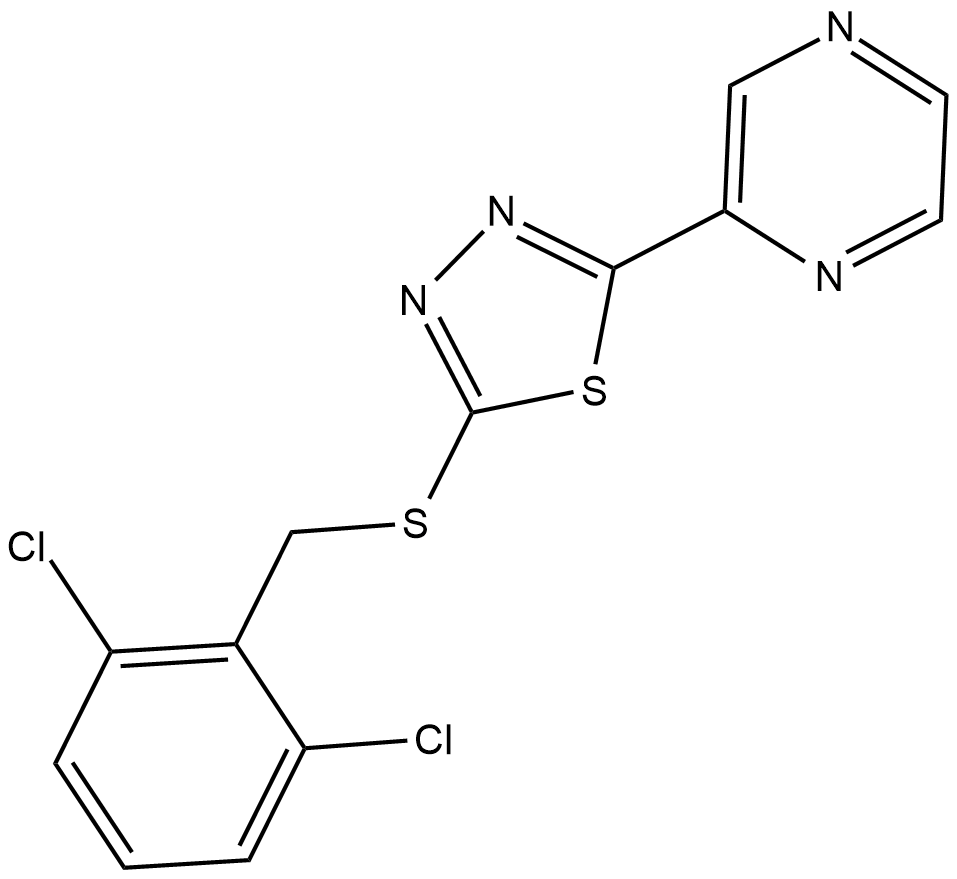
Yoda1is a synthetic and small molecule. Its molecular structure along with the functional groups involved in the PIEZO1 activation are given in Figure 1.
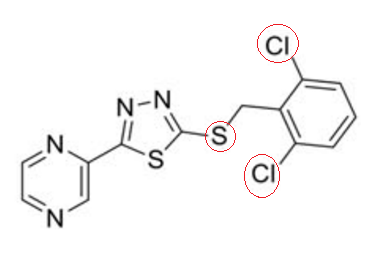
Figure 1 The 2D view of molecular structure of the Yoda1 compound. 2,6-Dichlorophenyl ring and thioether are the two functional group involved in the activation of PIEZO1 and encircled in red.
It selectively interacts and effectively activates PIEZO1 in micromolar range (10–50 µM) of concentration. PIEZO1 is a novel protein channel that is permeable to cation like Na+, K+ and Ca2+ etc. while being sensitive to and gets activated by the mechanical forces like membrane tension and the laminar flow of the biofluids thus more appropriately regarded as mechanosensitive in nature. As the mechanical force hits the human skin or tissue in microenvironment, the specialized protein PIEZO1 allows the flow of the above-mentioned ions especially Calcium ions (a signaling molecule) across the plasma membrane into the cell space along the concentration; thereby an electrical signal is produced as a response to the external mechanical stimuli. This effect is even observed in the lipid bilayer membrane without any cellular component in in vitro (Syeda et al., n.d.). This mechanosensitive activity of PIEZO1 could also be alternatively enhanced by small molecules like Yoda1.
In the absence of any external force, the PIEZO1 remains in a closed conformational state; it is a homotrimer (three identical molecules of PIEZO1 are bounded together) and its structure is more like a propeller having a central pore or pocket which is surrounded by three domains known as arms (also known as blades). Each one of these arms or blades is structured by helices known as Piezo repeats. The arms or blades are bounded to a rode extending through the membrane to the intracellular environment; they are proposed to affect the three-dimensional structure of the plasma membrane and thus act as mechanosensitive. As an external force hits the lipid bilayer, PIEZO1 changes its conformation to open state and gets activated. The conformational states (close and open), along with proposed directional movements leading to the conformational change, and proposed channel space for cationic current are represented in Figure 2 although exact molecular mechanism underlying this conformational change is still unknown.
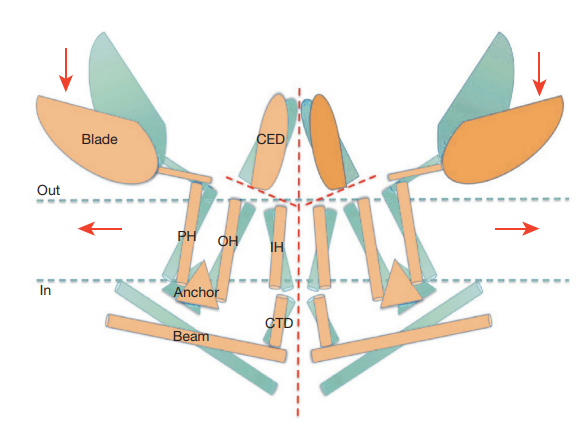
Figure 2 The conformational states, i.e., close and open, etc. of a PIEZO1 molecule are represented in blue and orange. It is proposed that pressure trigger conformational changes in the PIEZO1 molecule in the directions indicated by red arrows and the resultant cation ionic current travels through the space indicated by a central dash line in red.
In the PIEZO1 structure (as shown in Figure 3, Figure 4, Figure 5 and Figure 6), the peripheral arms or blades act as force sensor while the central pore or pocket acts as cation conducting. Its primary structure (including the number as well as the linear sequence of amino acids) has not accurately known in human yet, but its length is 2,547 amino acids (aa) in the mice. For mammalian, a close estimate of 2,500 amino acid residues is presented so far.
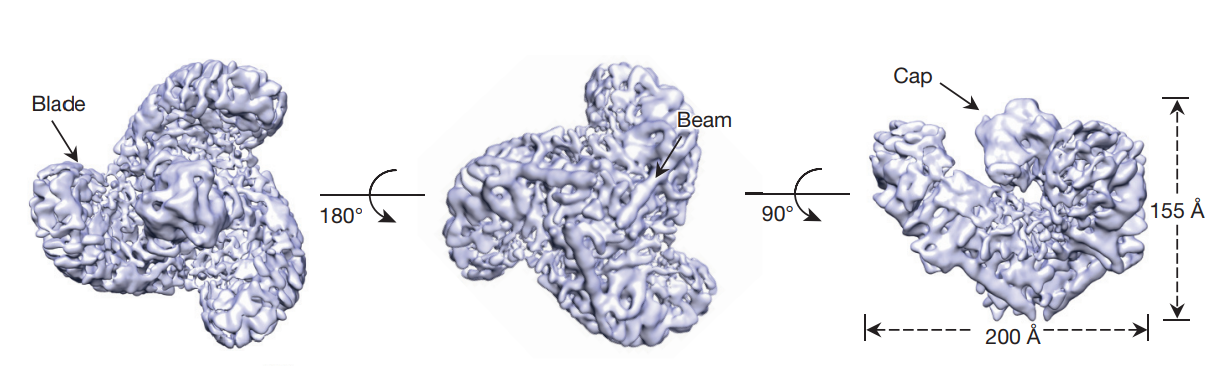
Figure 3 Three arms/ blades in Top view (left image), beams in Bottom view (middle image) and a cap in Side view (right image) of a PIEZO1 molecule are labelled. The dimensions of a PIEZO1 molecule are also indicated.
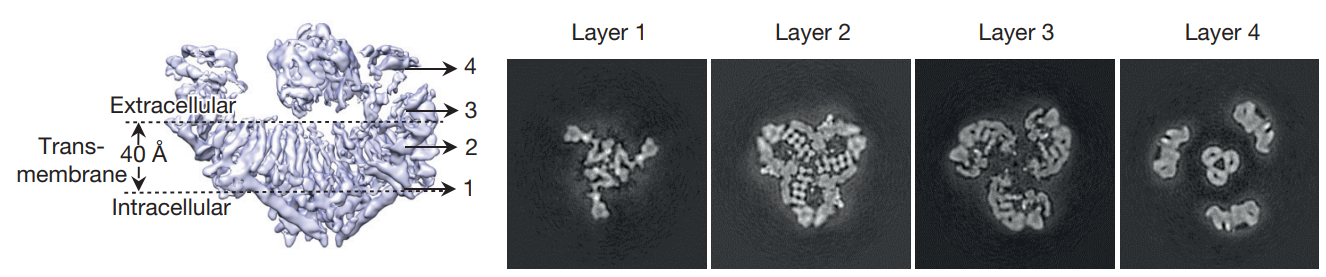
Figure 4 The extracellular region, transmembrane region and intracellular region of a PIEZO1 molecule are indicated (in leftmost image) and the four 2D slices are shown as four layers in right.
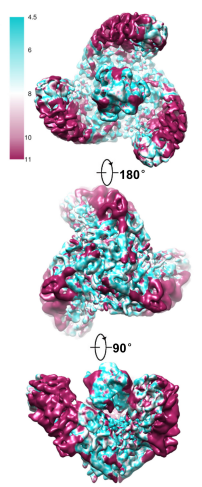
Figure 5 The colour-scaled density map of a PIEZO1 molecule given in top view, bottom view and side view, respectively.
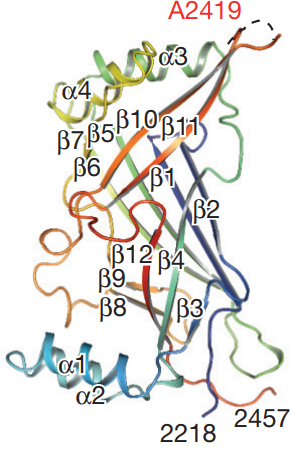
Figure 6 The extracellular domain of a PIEZO1 molecule. Only the C terminal segment is shown.
PIEZO1 protein is abundantly expressed in the cells of the skin, bones, lungs, colon, kidney and bladder. Physiologically, PIEZO1 regulates sensation of touch and hearing, blood pressure, maturation of vasculature in embryo, performance of the physical activities, hypertension-dependent structural remodelling in the arteries, osmoregulation via triggering the urination, homeostasis in the epithelium, growth of axons, volume of the red blood cells, proprioception, visceral and somatic mechanosensation,
There exist certain pathological conditions in case of dysfunctional PIEZO1 (for example lymphedema, arthrogryposis, Sleep apnea, Allodynia, abnormal development of vasculature in embryo) and either type of mutation (loss of function and gain of function) in PIEZO1. Lymphatic dysplasia a congenital disease that is autosomal recessive in nature happens in case of loss of function mutation in PIEZO1 while haemolytic anaemia also known as hereditary stomatocytosis or Hereditary xerocytosis (HX) happens in case of gain of mutation in PIEZO1.
Yoda1 can also increase the formation of calcium and nitrate ions in the endothelial cells (ECs) independent of PIEZO1 mechanosensitive activity. Yoda1 promotes the expression of two signaling protein which are usually activated by the blood flow and the intracellular calcium ions, i.e., Akt (as shown in Figure 7) and ERK1/2 (as shown in Figure 8), in the test cell line that is human coronary artery endothelial cells (HCAECs) in a dose-dependent manner. It could be inferred that the signaling pathways working downstream of Akt and ERK1/2 are activated in the Yoda1 treated cells. Both these signaling pathways regulate cell survival and proliferation .
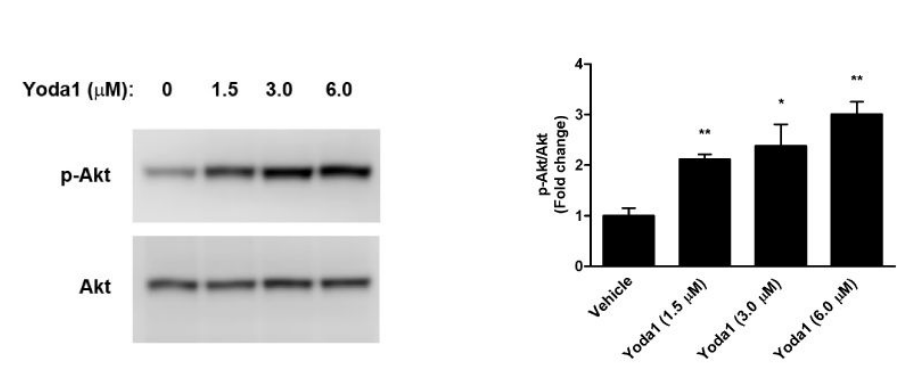
Figure 7 The Yoda1 treatment promotes the expression of Akt protein (thereby post-translationally modified phosphorylated form also exist which is p-Akt) in a dose-dependent manner as indicated by the increasing width and fluorescence of the protein band (in western blot), and increasing concentration of Akt protein (in right graph)
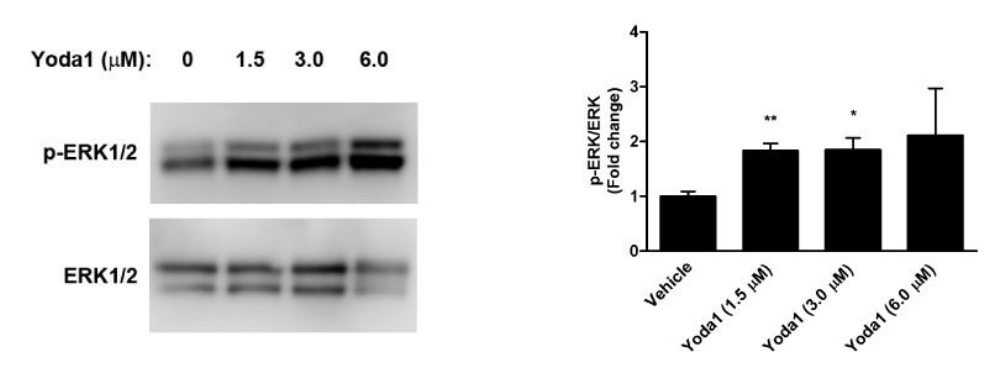
Figure 8 The Yoda1 treatment promotes the expression of ERK1/2 protein (thereby post-translationally modified phosphorylated form also exist which is p-ERK1/2) in a dose-dependent manner as indicated by the increasing width and fluorescence of the protein band (in western blot), and increasing concentration of Akt protein (in right graph)
The Rac1 activated by epidermal growth factor (EGF), leads to the formation of lamellipodia, ruffles and macropinosomes by deforming the cell membrane via actin rearrangement as shown in Figure 9 and Figure 10. Pathologically, this mechanism is exploited by the cancer cells for survival. Yoda1 inhibits the Rac1 activation and thereby the actin rearrangement via actin polymerization, and resultantly formation of lamellipodia, ruffles and macropinosomes is also prevented as summarized in Figure 10.
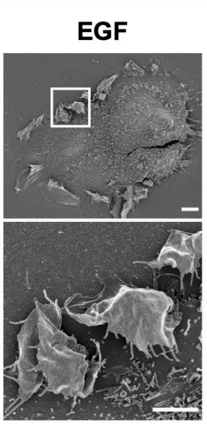
Figure 9 Micrograph of the cells having epidermal growth factor (EGF) activated Rac1
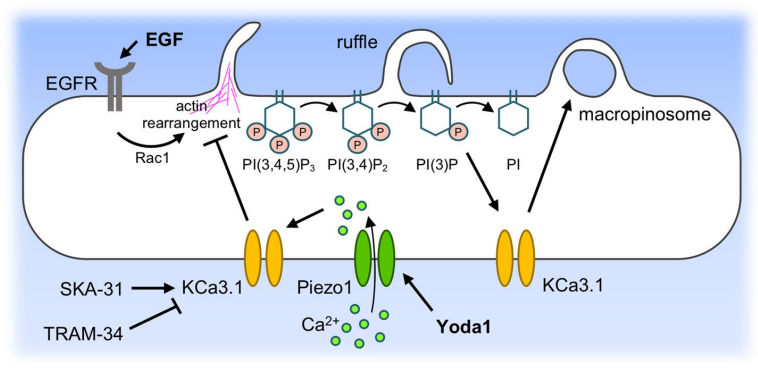
Figure 10 Mechanism of Inhibiting Rac1 via Yoda1
Apart from being a therapeutically relevant drug in the clinical settings, Yoda1 has also been used in exploring whether a given medical condition which is macroscopically initiated and aggravated by the applied mechanical pressure is involving PIEZO1 activation or not. This type of studies helps in determining whether PIEZO1 would be an ideal drug target for the treatment of that clinical condition; thus, Yoda1 could be used in research applications where the PIEZO1 activation has to be confirmed as the underlying mechanism of disease initiation and prognosis. Studies based on similar format has been useful in confirming the pathological role of PIEZO1 in a couple of diseases, i.e., sickle cell anaemia (SCA) and acute pancreatitis.













コメント