Oritavancin (phosphate) (Synonyms: LY 333328) |
| カタログ番号GC44515 |
オリタバンシン (リン酸) (LY333328 二リン酸) は、グラム陽性生物に対する活性を有する経口活性糖ペプチド抗生物質です。
Products are for research use only. Not for human use. We do not sell to patients.
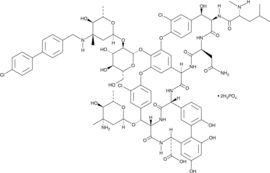
Cas No.: 192564-14-0
Sample solution is provided at 25 µL, 10mM.
Oritavancin is a semisynthetic glycopeptide antibiotic that inhibits the growth of Gram-positive bacteria. It inhibits transglycosylation and transpeptidation in the bacterial cell wall to disrupt membrane integrity. Oritavancin inhibits the growth of clinical isolates from skin and soft-tissue infections, including methicillin-resistant and -susceptible S. aureus (MRSA, MSSA), vancomycin-resistant and -susceptible E. faecium (VREF, VSRF), as well as other staphylococci, streptococci, and enterococci (MIC90s = ≤0.008-0.5 mg/L). Oritavancin inhibits growth of S. aureus in a neutropenic-mouse thigh model of infection (ED50 = 0.95 mg/kg for a single dose). It improves survival in a mouse inhalation model of B. anthracis Ames anthrax when administered prophylactically pre- and postexposure and when administered post symptom development. Formulations containing oritavancin have been used to treat bacterial skin infections.
Average Rating: 5 (Based on Reviews and 3 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















