Dabrafenib (GSK2118436) (Synonyms: GSK2118436) |
| カタログ番号GC15187 |
Dabrafenib (GSK2118436) (GSK2118436A) は、C-Raf および B-RafV600E に対してそれぞれ 5 nM および 0.6 nM の IC50 を持つ Raf の ATP 競合阻害剤です。
Products are for research use only. Not for human use. We do not sell to patients.
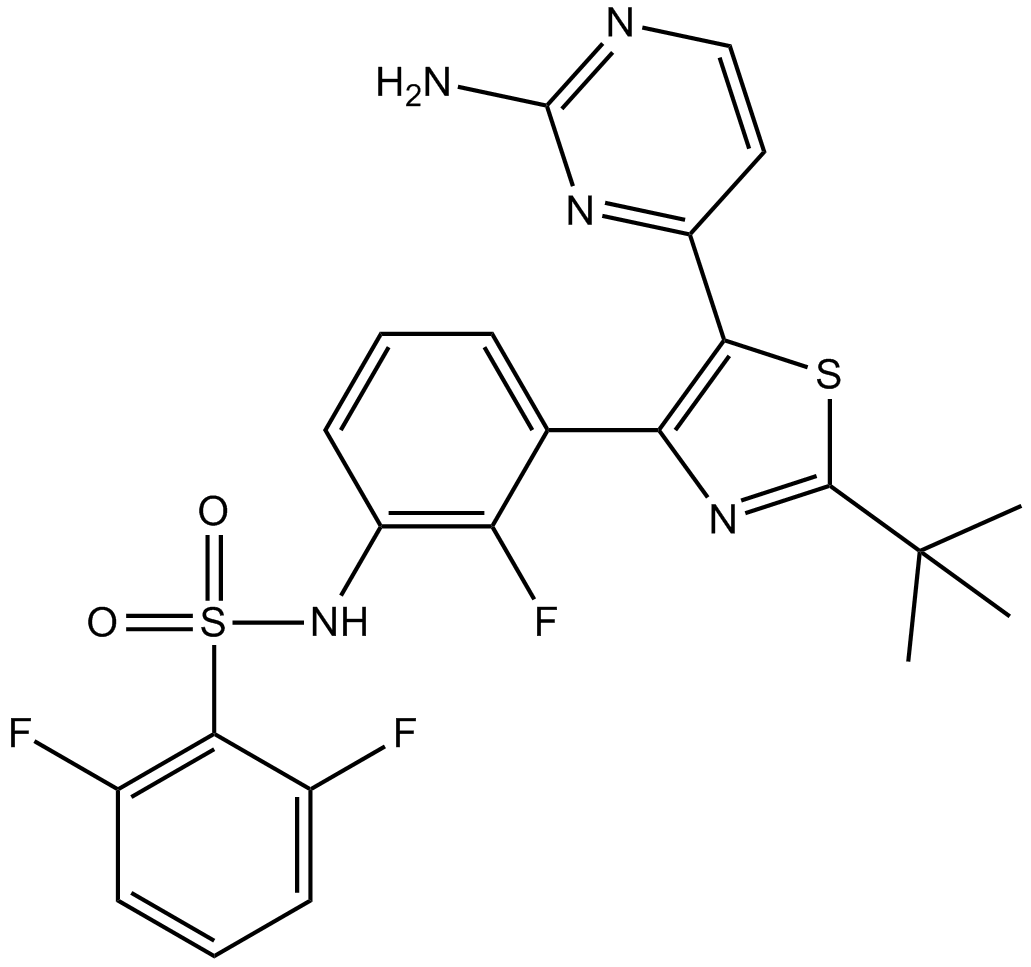
Cas No.: 1195765-45-7
Sample solution is provided at 25 µL, 10mM.
Dabrafenib is a specific inhibitor of BRAF V600 mutants with IC50 values of 0.5nM, 0.6nM and 1.9nM against V600E, V600K and V600D, respectively [1].
BRAF plays a central role in regulating MAPK signaling pathway which regulates cell growth, division and differentiation. The V600E mutation of BRAF increases the kinase activity and is involved in metastatic melanomas. Dabrafenib is an ATP-competitive and reversible inhibitor of BRAF mutants. It potently inhibits BRAFV600E, BRAFV600K and BRAFV600D with IC50 values of 0.5nM, 0.6nM and 1.9nM, respectively. Dabrafenib is currently approved by FDA and is widely used in cancer patients harboring BRAF mutations. It is reported that treatment of dabrafenib shrinks the overall size of brain metastases in patients. It also has an impressive 60% response rate for melanomas outside of the brain. Dabrafenib provides a significant survival benefit in patients with metastatic melanoma [1, 2].
References:
[1] Hong S, Hong S. Overcoming metastatic melanoma with BRAF inhibitors. Archives of pharmacal research, 2011, 34(5): 699-701.
[2] Hong D S, Vence L, Falchook G, et al. BRAF (V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clinical Cancer Research, 2012, 18(8): 2326-2335.
Average Rating: 5 (Based on Reviews and 28 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















