Dabrafenib Mesylate (GSK-2118436) (Synonyms: GSK2118436B) |
| カタログ番号GC12486 |
ラフキナーゼ阻害剤
Products are for research use only. Not for human use. We do not sell to patients.
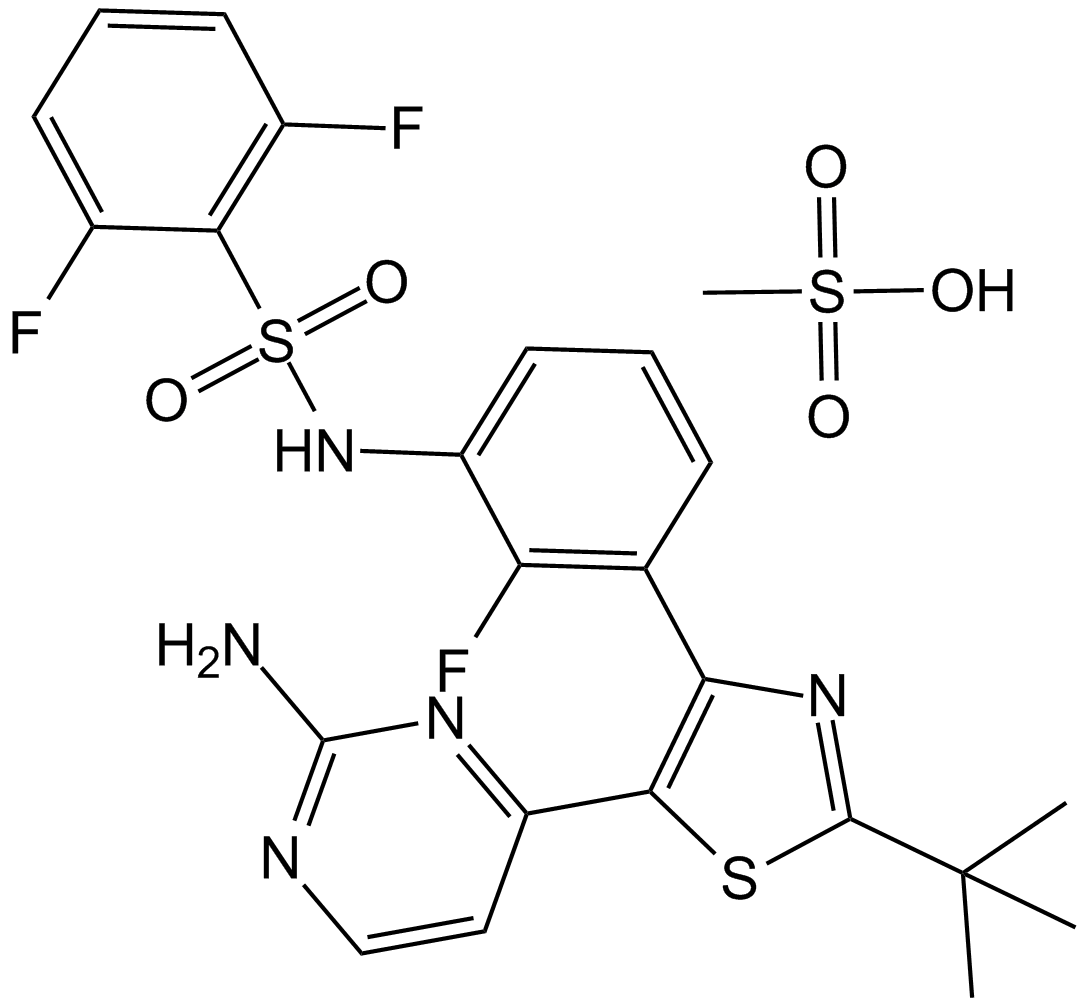
Cas No.: 1195768-06-9
Sample solution is provided at 25 µL, 10mM.
GSK2118436 is a selective BRAF V600E inhibitor. BRAF encodes a proto-oncogene B-Raf also known as serine/threonine protein kinase B-Raf. It is critical in regulating the MAPK/ERK signaling pathway. BRAF mutations frequently occur in many human cancers. [1, 2] BRAF V600E mutant is constitutively active, allowing MAPK/ERK activation independent of upstream cues. [3]
GSK2118436 binds to Raf family kinases and inhibits their activity. It is highly selective against B-Raf V600E with IC50 of 0.8 nM, compared to wild type B-Raf and c-Raf with IC50s of 3.2 nM and 5.0 nM, respectively. [4]
GSK2118436 treatment shows selective inhibition of MAPK/ERK activation, proliferation, transformation and tumorigenicity. FDA approved GSK2118436 as a single agent treatment for advanced melanoma with BRAF V600E mutation on May 30, 2013.
GSK2118436 can be taken orally.
References:
[1]Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T, Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 2003; 88 (9): 4393–7.
[2]Tan YH, Liu Y, Eu KW, Ang PW, Li WQ, Salto-Tellez M, Iacopetta B, Soong R. Detection of BRAF V600E mutation by pyrosequencing. Pathology 2008; 40 (3): 295–8.
[3]Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417: 949-954.
[4]Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, Lazova R, Klump V, Pawelek JM, Xu X, Xu W, Schuchter LM, Davies MA, Herlyn M, Winkler J, Koumenis C, Amaravadi RK. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014; 124(3): 1406-17.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















