Paritaprevir (Synonyms: ABT-450) |
| カタログ番号GC19275 |
パリタプレビル (ABT-450) は、HCV 1a および 1b に対する EC50 がそれぞれ 1 および 0.21 nM である、強力な経口活性のある抗ウイルス性の非構造タンパク質 3/4A (NS3/4A) プロテアーゼ阻害剤です。
Products are for research use only. Not for human use. We do not sell to patients.
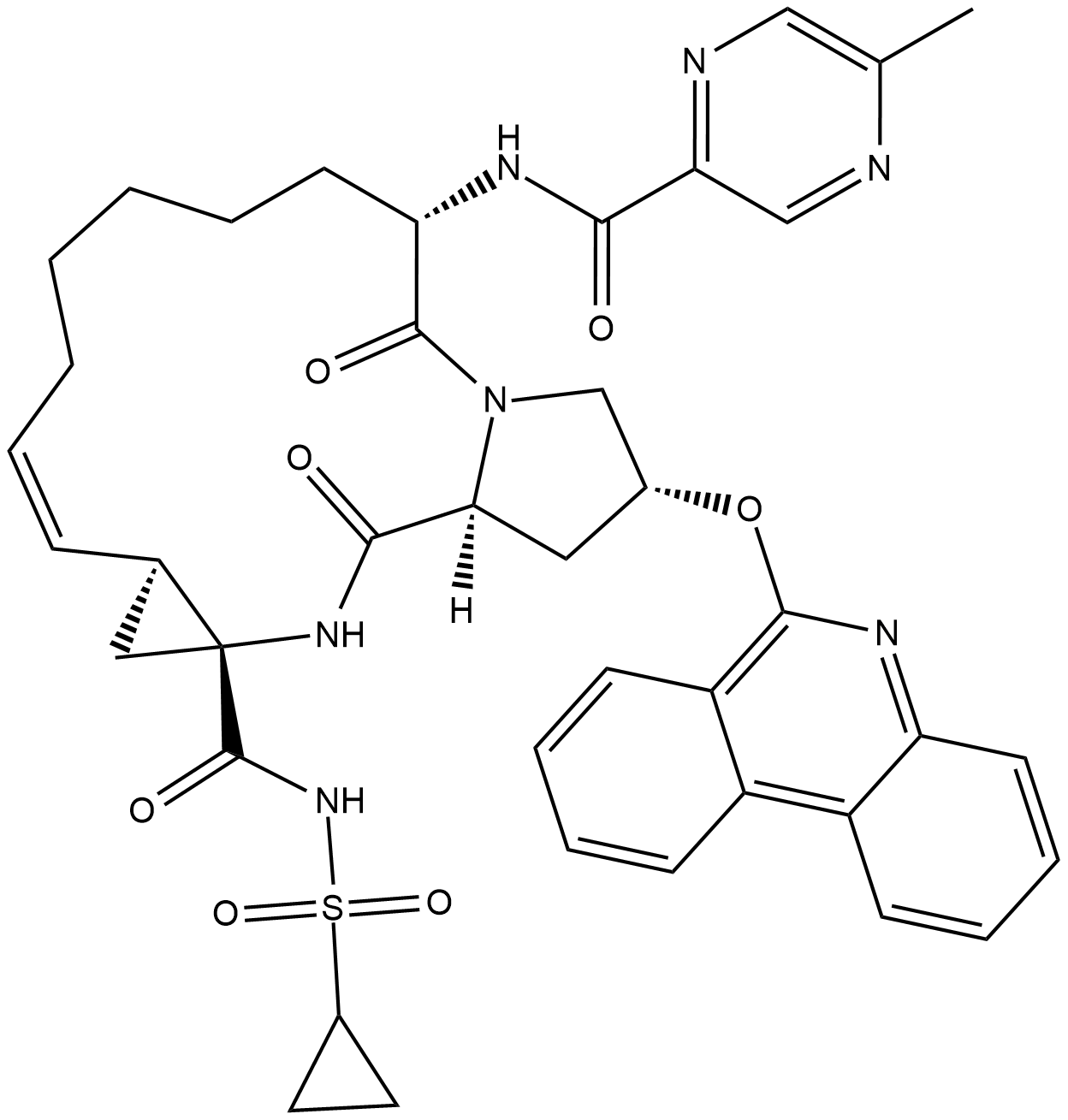
Cas No.: 1216941-48-8
Sample solution is provided at 25 µL, 10mM.
Paritaprevir (ABT-450) is a potent non-structural protein 3/4A (NS3/4A) protease inhibitor with EC50s of 1 and 0.21 nM against HCV 1a and 1b, respectively.
Paritaprevir demonstrates in vitro antiviral activity against HCV GT1-4 and GT6 (EC50 range, 0.09 to 19 nM), with an EC50 of 0.09 nM against GT4a[2].
The combination of paritaprevir, ritonavir, ombitasvir (an NS5A protein inhibitor), and dasabuvir (an NS5B non-nucleoside polymerase inhibitor) with or without RBV has been approved to treat HCV genotype 1 infections1[1].
References:
[1]. Smith MA,et al. Profile ofparitaprevir/ritonavir/ombitasvir plus dasabuvir in the treatment of chronic hepatitis C virus genotype 1 infection. Drug Des Devel Ther.2015 Nov 13;9:6083-94.
[2]. Schnell G, et al. Hepatitis C Virus Genotype 4 Resistance and Subtype Demographic Characterization of Patients Treated with Ombitasvir plus Paritaprevir/ritonavir. Antimicrob Agents Chemother. 2015 Aug 17. pii: AAC.01229-15.
Average Rating: 5 (Based on Reviews and 7 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















