Telbivudine (Synonyms: 2'-deoxy-L-Thymidine, L-Deoxythymidine, NV 02B, β-L-Thymidine) |
| カタログ番号GC11295 |
経口活性チミジンヌクレオシド類似体であるテルビブジン (エパブジン) は、B 型肝炎ウイルス (HBV) 複製の強力な抗ウイルス阻害剤です 。
Products are for research use only. Not for human use. We do not sell to patients.
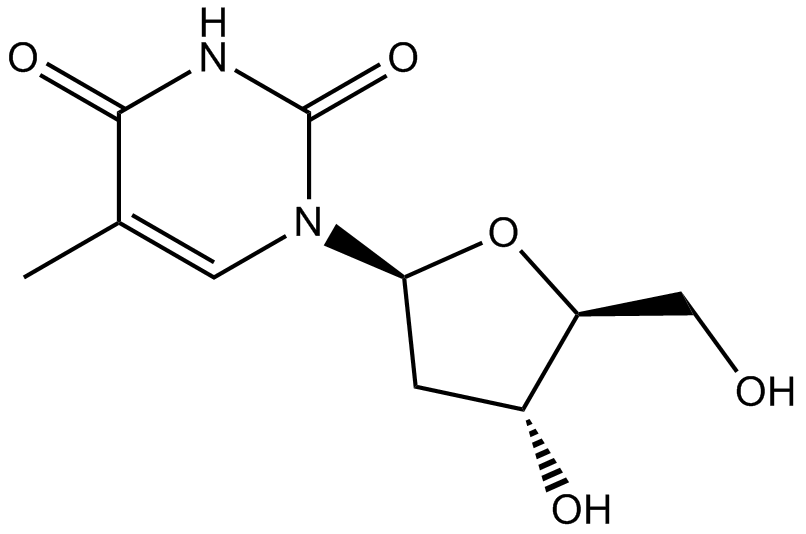
Cas No.: 3424-98-4
Sample solution is provided at 25 µL, 10mM.
Telbivudine, a specific inhibitor of hepatitis B virus (HBV) replication, is an antiviral drug used in the treatment of hepatitis B infection.Target: HBVTelbivudine is an antiviral drug used in the treatment of hepatitis B infection. It is marketed by Swiss pharmaceutical company Novartis under the trade names Sebivo (Europe) and Tyzeka (United States). Clinical trials have shown it to be significantly more effective than lamivudine or adefovir, and less likely to cause resistance. Telbivudine is a synthetic thymidine nucleoside analogue, it is the L-isomer of thymidine. It is taken once daily.Telbivudine is a potent antiviral that provides effective and sustained viral suppression in patients with compensated CHB. In clinical trials, treatment outcomes were improved significantly more with telbivudine 600 mg once daily than with lamivudine 100 mg or adefovir 10 mg once daily, and telbivudine-treated patients had significantly less viral resistance than lamivudine-treated patients. Telbivudine is associated with a medium genetic barrier to resistance and, as patients with undetectable HBV DNA levels have significantly improved outcomes, it is recommended that HBV DNA levels are monitored at week 24 (and 6 monthly thereafter), with the addition of a nucleoside/nucleotide analogue without cross resistance (such as adefovir dipivoxil) if viraemia is present to reduce the risk of resistance (Roadmap concept). Telbivudine was generally well tolerated in clinical trials for periods of up to 4 years, and has a similar tolerability profile to that of lamivudine.
References:
[1]. Lai CL, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005 Aug;129(2):528-36.
[2]. Lai CL, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007 Dec 20;357(25):2576-88.
[3]. McKeage K, et al. Telbivudine: a review of its use in compensated chronic hepatitis B. Drugs. 2010 Oct 1;70(14):1857-83.
Average Rating: 5 (Based on Reviews and 16 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















