Vodobatinib (Synonyms: K0706) |
| カタログ番号GC62103 |
ボドバチニブ (K0706) は、7 nM の IC50 を持つ強力な第 3 世代の経口活性 Bcr-Abl1 チロシンキナーゼ阻害剤です。ボドバチニブは、ほとんどの BCR-ABL1 点変異体に対して活性を示し、BCR-ABL1T315I に対しては活性を示しません。ボドバチニブは、慢性骨髄性白血病 (CML) の研究に使用できます。
Products are for research use only. Not for human use. We do not sell to patients.
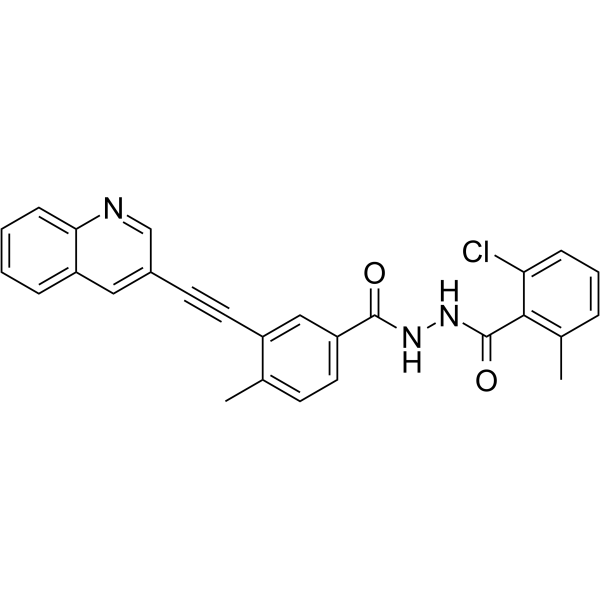
Cas No.: 1388803-90-4
Sample solution is provided at 25 µL, 10mM.
Vodobatinib (K0706) is a potent, third generation and orally active Bcr-Abl1 tyrosine kinase inhibitor with an IC50 of 7 nM. Vodobatinib exhibits activity against most BCR-ABL1 point mutants, and has no activity against BCR-ABL1T315I. Vodobatinib can be used for chronic myeloid leukemia (CML) research[1][2].
In Ba/F3 cells expressing BCR-ABL1, BCR-ABL1L248V, BCR-ABL1Y253H, or BCR-ABL1E255V, Vodobatinib (K0706; 0-2000 nM) treatment shows potent inhibition of BCR-ABL1 tyrosine autophosphorylation[1].
[1]. Orlando Antelope, et al. BCR-ABL1 tyrosine kinase inhibitor K0706 exhibits preclinical activity in Philadelphia chromosome-positive leukemia. Exp Hematol. 2019 Sep;77:36-40.e2.
[2]. Phase 1 Trial of Vodobatinib, a Novel Oral BCR-ABL1 Tyrosine Kinase Inhibitor (TKI): Activity in CML Chronic Phase Patients Failing TKI Therapies Including Ponatinib. Session: 632: Chronic Myeloid Leukemia: Therapy: CML: New and Beyond.
Average Rating: 5 (Based on Reviews and 12 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















