FK866
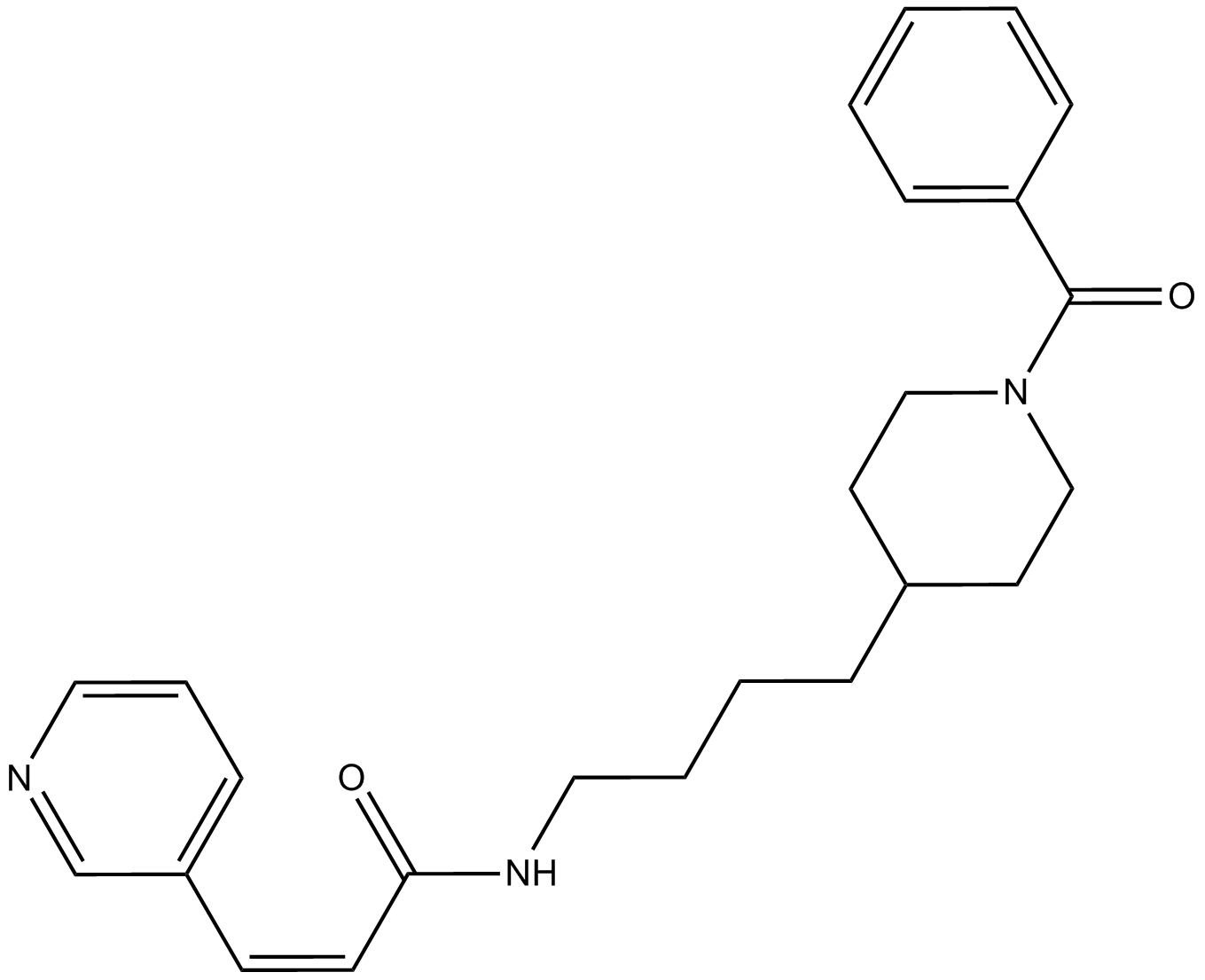
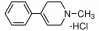
The full chemical name of the FK866 compound is (E)-N-[4-(1-benzoylpiperidin-4-yl) butyl]-3-(pyridin3-yl) acrylamide. It is also known as (E)-Daporinad and APO866 in the scientific literature. It is a small molecule with low molecular weight, artificially synthesized in laboratory and possess an entirely novel mechanism for its pharmaceutical action. A complete picture two-dimensional view of the molecular structure of the FK866 compound is presented in Figure 1.
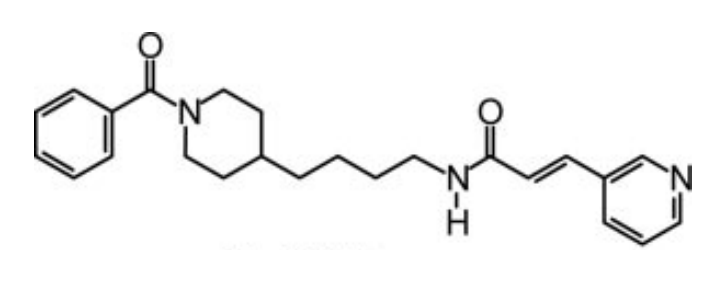
Figure 1 The two-dimensional view of the molecular structure of the FK866 compound
The FK866 molecule specifically interacts and effectively inhibits nicotinamide phosphoribosyltransferase (NAPRT) without exhibiting any primary impact on the energy yielding biochemical pathways, i.e., cellular metabolism. This inhibition gradually leads to the extremely decreased levels of Nicotinamide Dinucleotide (NAD+) within the mitochondria and thus no energy, i.e., ATP as this gets synthesized from NAD, could be provided by the powerhouse of the cell; with these less levels of Nicotinamide Dinucleotide (NAD+), a cell cannot grow and thus could not reproduce and not even survive. In other words, nicotinamide phosphoribosyltransferase (NAPRT) can be understood as rate limiting enzyme, i.e., an enzyme that catalyzes the rate determining step of the overall biosynthesis of Nicotinamide Dinucleotide (NAD+); thus, controlling the biosynthesis of Nicotinamide Dinucleotide (NAD+). In that case, the FK866 treated cells shift towards the production of Reactive Oxygen Species (ROS) that ultimately leads to the onset of a programmed cell death namely apoptosis.
As the name indicates, nicotinamide phosphoribosyltransferase (NAPRT) is an enzyme, i.e., a linear structure of a large number of covalently bounded residual amino acids having N-terminal (amino group) and a C-terminal (carboxyl group) organized into tertiary level of protein structure and thus globular in nature. Being a protein, it would be formed through the gene expression. The nicotinamide phosphoribosyltransferase (NAPRT) is expressed from the mitochondrial DNA and present in the mitochondrial matrix; thus, it is more appropriately regarded as a mitochondrial enzyme.
Talking about the structural features of the nicotinamide phosphoribosyltransferase (NAPRT) enzyme, the primary structure of the nicotinamide phosphoribosyltransferase (NAPRT) enzyme is composed of a linear sequence of covalently bonded 491 residual amino acids via the peptide linkages as presented in Figure 2. The secondary structure of the nicotinamide phosphoribosyltransferase (NAPRT) enzyme is composed of both alpha-helices and beta-strands, i.e., 22 beta-strands and 15 alpha-helices which could be seen in orange and cyan in Figure 2. The 3D crystal/ globular/ tertiary structure of one molecule and two molecules of the nicotinamide phosphoribosyltransferase (NAPRT) enzyme is presented in Figure 3 and Figure 4 as determined by selenomethionyl SAD method, respectively.
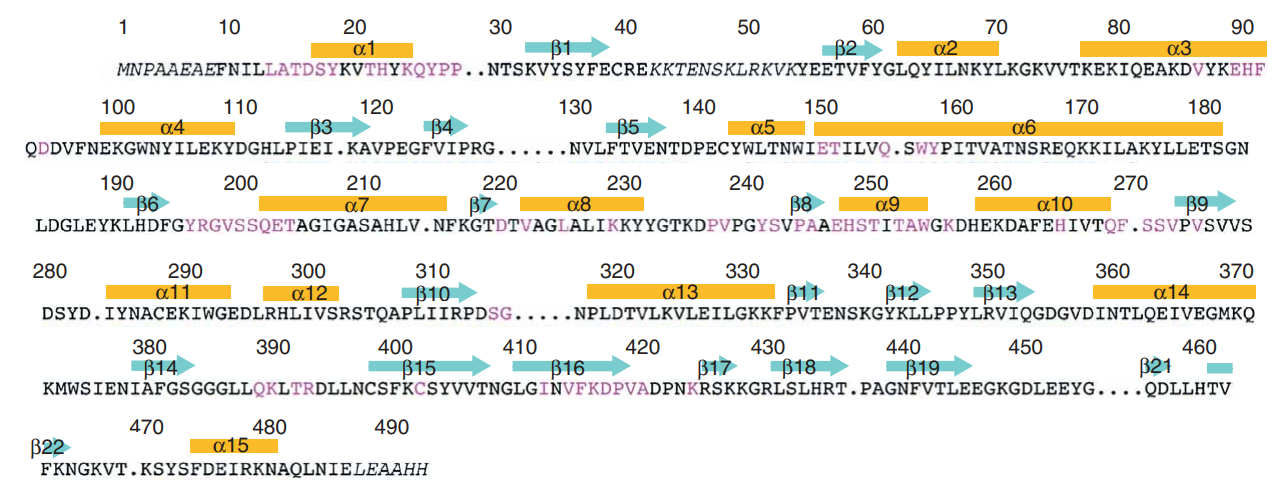
Figure 2 The incomplete primary structure but the complete secondary structure of the nicotinamide phosphoribosyltransferase (NAPRT) enzyme. Some omitted amino acids within the primary structure residues are indicated with dots while all the alpha-helices and beta-strands within the secondary structure are indicated by orange bars and cyan arrows, respectively.
Taking about the functional domains of the nicotinamide phosphoribosyltransferase (NAPRT) enzyme, it contains three distinct functional domains namely Domain 1, Domain 2 and Domain 3. Talking about the structural features of each of these domains; Domain 1 encompasses a b-sheet which is composed of seven strands arranged in an antiparallel fashion, along with five helices. In terms of the residual amino acids, it contains 9–148, 391–427 and 459–484. Domain 2 encompasses a beta/alpha core having residuals 181–390. Domain 1 and Domain 2 are connected via Helix alpha6 having residuals 149–180. and Domain 3 encompasses a beta-sheet which is composed of three strands arranged in an antiparallel fashion having 428–458 residuals.
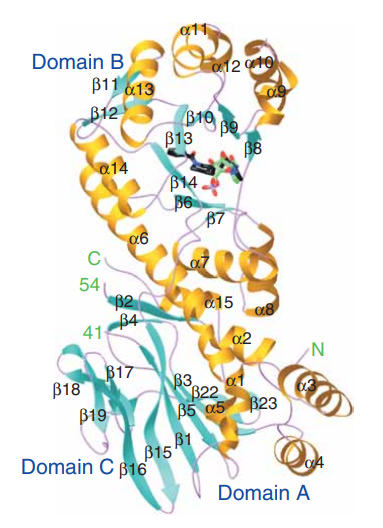
Figure 3 The 3D crystal/ globular structure of one molecule (monomer) of the NAPRT in which the arrangement of both alpha-helices which are in orange and beta-strands which are in cyan could be observed.
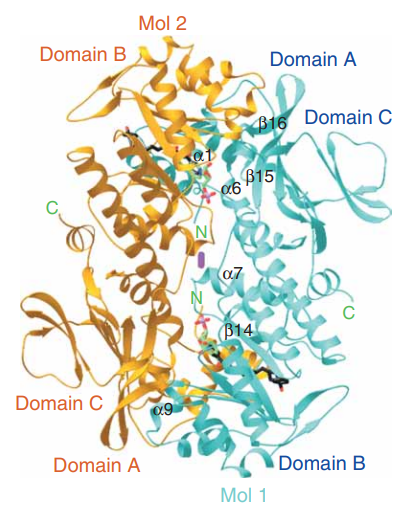
Figure 4 The 3D crystal/ globular structure of two molecules (as a dimer that the stable form in an aqueous solution) of the NAPRT in which the arrangement of two individual molecules could be observed. One molecule is labelled as Mol1 and presented in cyan while the other molecule is labelled as Mol2 and presented in orange.
In cell physiology, nicotinamide phosphoribosyltransferase (NAPRT) is expressed in almost every organ of the human body, for example, adipose tissue, blood, liver tissue, cerebrospinal fluid, pancreatic tissue, etc. and acts as a key molecular player in the cell metabolic pathway that is involved in the biosynthesis of Nicotinamide Dinucleotide (NAD+) which has the capability to act as a coenzyme and also a substrate/ reactant in biochemical reactions, from Nicotine molecules.
In certain pathological condition, i.e., various types of cancers/ neoplasms, overexpression of NAPRT is a common clinical feature namely melanoma (cancer of skin), myeloma (cancer of plasma cells of blood), b-cell lymphoma (cancer of the lymphocytes of the lymph), glioma (cancer of glial cells of brain and spinal cord), gastric cancer (cancer of the alimentary canal/ gastric tract), colorectal cancer (cancer of the colon and rectum regions of the whole digestive system), prostate cancer in male individuals and, breast cancer and ovarian cancer in female individuals. In the cancer cells that are also known as tumors, NAD+ is produced as well as consumed in greater amounts than the normal cells. Other than neoplasm, there are other pathological conditions as for example, non-alcoholic fatty liver disease, obesity and diabetes, etc. which are marked by the overexpression of nicotinamide phosphoribosyltransferase (NAPRT). Thus, controlling the amount of Nicotinamide Dinucleotide (NAD+) would be a promising target for the treatment of the above-mentioned cancer types as well as for the noncancerous pathological conditions.
The FK866 compound is the proposed inhibitor that gets placed in the center of the beta sheet of the Domain B where there is a pocket formed as a result of homodimerization. The pyridyl ring of the FK866 compound gets placed between the Phe193 and Tyr18’. The carbonyl oxygen atom of the amide bond of the FK866 compound is hydrogen bonded to the hydroxyl of Ser275 while the nitrogen atom is hydrogen bonded to a water molecule. The carbon backbone of FK866 compound mostly interacts with the hydrophobic side chains in the b-sheet of domain B. The phenyl ring of the FK866 compound gets placed in a shallow groove of nicotinamide phosphoribosyltransferase (NAPRT). When the FK866 molecule binds to nicotinamide phosphoribosyltransferase (NAPRT), just the side chains of Tyr240 and Tyr18’ undergo conformational changes. All these interactions lead to the inhibition of nicotinamide phosphoribosyltransferase (NAPRT) and are outlined in Figure 5, Figure 6 and Figure 7. (Khan et al., 2006)
Figure 5 The electron density map of the FK866 molecule
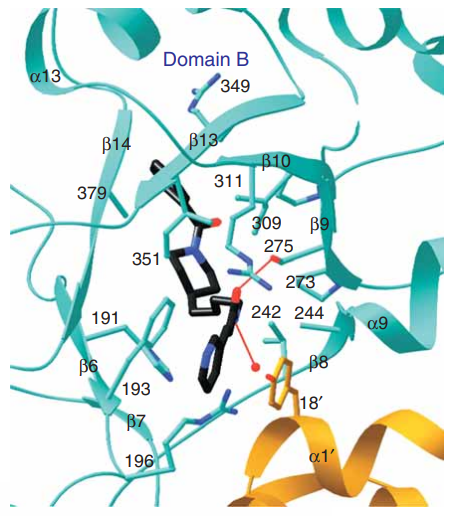
Figure 6 The FK866 compound is bounded to the nicotinamide phosphoribosyltransferase (NAPRT) at the interface of its homodimer. One molecule is shown in blue, while other one is in orange. A red sphere indicates the water molecule.
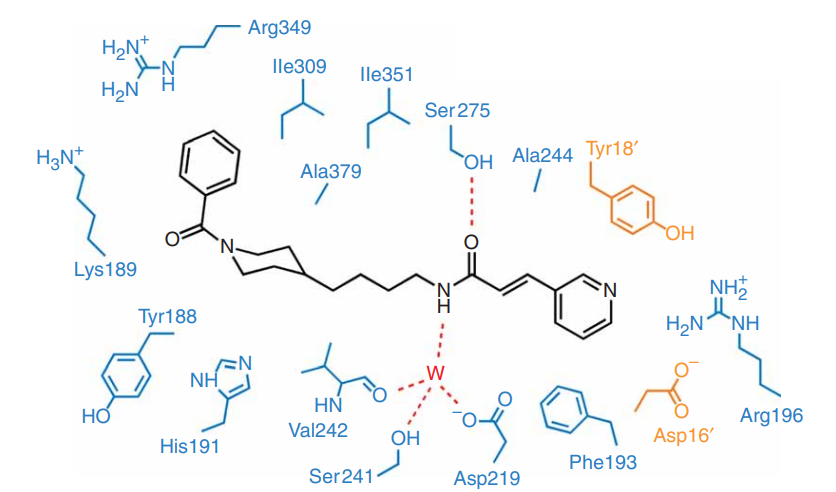
Figure 7 The intermolecular forces between the FK866 compound and the residual amino acids of the nicotinamide phosphoribosyltransferase (NAPRT) homodimer are shown by the dashed lines are actually the hydrogen bonds. Blue residual amino acids belong to one molecule of NAPRT homodimer while orange residual amino acids belong to the other molecule of NAPRT homodimer. The letter W represents the same water molecule that is represented by red sphere in Figure 6












Comments