Rapamycin (sirolimus)
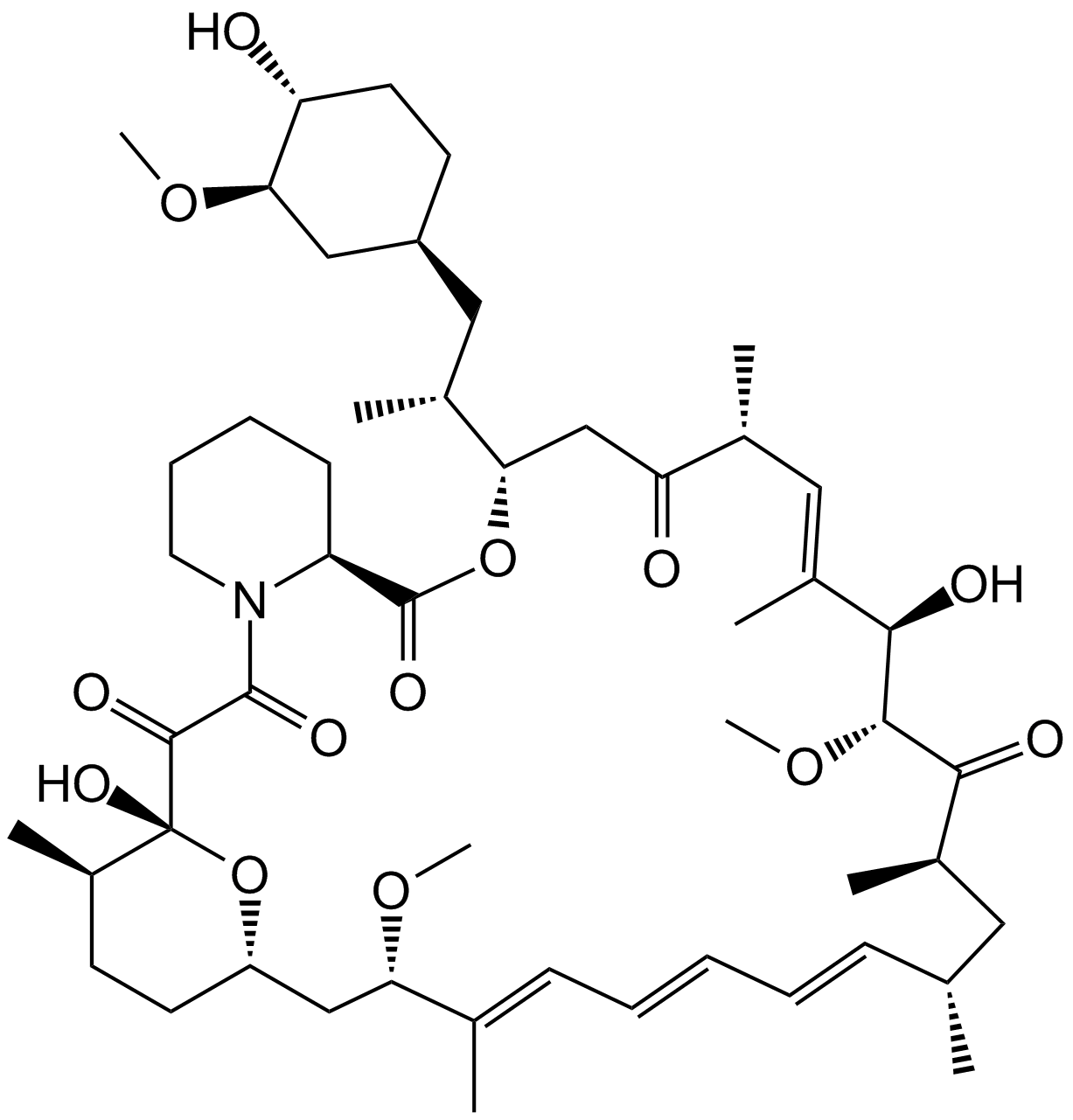
Rapamycin is also known as sirolimus. It is made by the bacteria Streptomyces hygroscopicus, which was initially isolated from samples discovered on Easter Island in 1972. Rapamycin was the initial name of the chemical, taken from the Rapa Nui, the local name of the island. The molecular structure of rapamycin compound is depicted in Figure1.
Figure 1: The 2D view of the molecular structure of Rapamycin compound
Rapamycin, has since been found to possess immunosuppressive and antiproliferative properties in mammalian cells. This macrolide drug is the first and currently the only drug approved by the US Food and Drug Administration that directly inhibits the mammalian target of rapamycin (mTOR) complex 1 (mTORC1). As demonstrated by several studies, rapamycin is a strong inhibitor of S6K1 activation, a serine/threonine kinase that is activated by a range of agonists, and a crucial mediator of PI3 kinase signaling . Meanwhile, Rapamycin's (TOR) target in yeast and animals cells has been found. Rapamycin creates a gain-of-function complex with the 12 kDa FK506-binding protein (FKBP12), which binds to and selectively inhibits mammalian TOR complex 1 (mTORC1) (also known as mechanistic TOR). Analysis of mTOR's biochemistry and genetics has shown that it exists in two functionally different complexes. mTORC1's essential components are mTOR, mammalian lethal with sec-13 protein 8 (mLST8), and TOR regulatory-associated protein (raptor). DEP-domain-containing mTOR-interacting protein (DEPTOR) and proline-rich Akt substrate 40 kDa (PRAS40) are also present. The rapamycin insensitive partner of mTOR (rictor), stress-activated protein kinase-interacting protein 1 (mSIN1), and mLST8 comprise the mTOR complex 2 (mTORC2) core. Additional regulatory components found with rictor 1/2 (protor 1/2) and DEPTOR . Two well established mTORC1 substrates are S6 kinase 1 (S6K1) and eukaryotic inhibitory factor eIF4E binding protein 1. Only mTORC1 is acutely responsive to rapamycin inhibition. Long-term rapamycin exposure, on the other hand, inhibits mTORC2 in particular cell types by sequestering newly produced mTOR molecules . mTORC1 is a signal integrator that responds to numerous signals from growth factors, nutrients, energy, and oxygen status to control cell growth and proliferation activities such as mRNA biogenesis, protein, lipid, and nucleotide synthesis, energy metabolism, including autophagy (Figure 2).
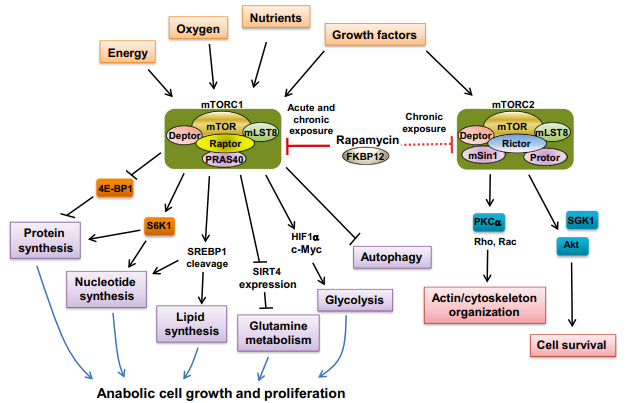
Figure 2: The two mTOR complexes underlie the regulation of critical cellular processes
Many human malignancies have increased mTORC1 activation due to gain-of-function mutations in oncogenes (e.g., PI3K, AKT, or Ras) and/or loss-of-function mutations in tumor suppressors (e.g., PTEN, LKB1, or TSC1/2), which are upstream regulators of mTORC1. Cancer cells benefit from these alterations in terms of selective growth over normal cells . Cancer cells frequently exhibit basic changes in food absorption and energy metabolism, activities that are directly regulated by the mTORC1 pathway, in order to satisfy the high demands of proliferation. Thus, oncogenic activation of mTORC1 not only drives protein synthesis but also activates a gene expression pathway important in metabolic reprogramming of cancer cells. Activation of mTORC1 stimulates lipid biosynthesis and the pentose phosphate pathway through sterol regulatory element binding protein 1 (SREBP-1) , upregulates hypoxia-inducible factor alpha (HIF1a) and c-Myc, and positively regulates glutamine metabolism through SIRT4 repression . As a result, medications that specifically target mTORC1, such as rapamycin, are thought to potentially inhibit cancer metabolism.
A rare lung condition called lymphangieiomyomatosis (LAM) is typified by the aberrant proliferation of cells that resemble smooth muscle. An anti-inflammatory reaction, the development of cysts, and eventually respiratory failure can arise from the infiltration of the lung with these cells through an enigmatic process known as benign metastasis . Almost primarily affecting women of childbearing age, LAM can develop alone (sporadic LAM) or in conjunction with the tuberous sclerosis complex (TSC). A genetic condition known as TSC is typified by the growth of benign hamartomatous tumors that affect many organ systems. Loss-of-function mutations in the TSC genes (TSC1 or TSC2), whose protein products work as a complex to block the activity of mTORC1, are the cause of both LAM and TSC. Thus, the uncontrolled cell proliferation that causes the clinical symptoms is directly related to overactive mTORC1 signaling. Therefore, using mTORC1 inhibitors may be advantageous for patients with TSC and LAM. Over the previous few years, a number of clinical trials were carried out to assess the security and effectiveness of rapamycin-based therapy. In 2011, McCormack et al., for instance revealed a randomized, double-blind, placebo-controlled study in which included 89 LAM patients with significant lung impairment were allocated to either the sirolimus (rapamycin) or placebo for a year, then observe for another year. Their research showed that giving patients with LAM sirolimus for a year stabilized lung function and enhanced quality of life (Figure 3).
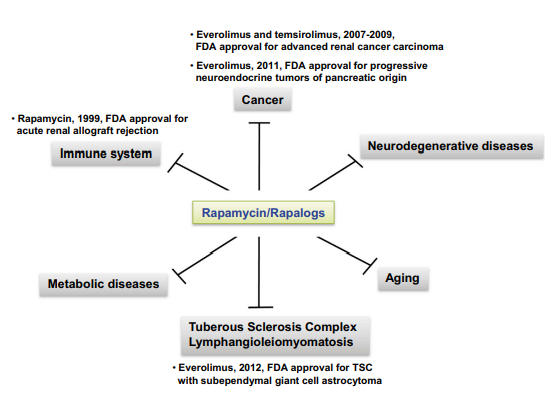
Figure 3: Effects of rapamycin in various diseases
However, after discontinuing sirolimus use, patients' Lung function declined once more. The writers deduced that to learn more about the length of treatment and long-term safety of sirolimus, more trials are needed . Angiomyolipomas (AMLs), which are benign kidney tumors, arise in around 80% of all TSC patients and 50% of women with LAM. Two independent trials were carried out by Bissler et al. to assess the efficacy of sirolimus or everolimus for AML in LAM or TSC. According to their findings, with sirolimus or everolimus, AML volume declined .
Rapamycin's positive benefits have mostly been demonstrated in preclinical animal studies. However, rapamycin's therapeutic efficacy has been linked to only a few benign and malignant tumors. The occurrence of numerous feedback loops involved in cell survival responses is one of the reasons for rapalogs' limited therapeutic success. Under normal conditions, mTORC1-mediated S6K1 activation increases the degradation of insulin receptor substrate (IRS) and the consequent down-regulation of PI3K signaling. Rapamycin inhibits mTORC1, breaking the negative feedback loop and boosting PI3K signaling. Similarly, mTORC1-mediated S6K1 signaling also inhibits mTORC2 through phosphorylation of rapamycin disrupts this feedback loop, resulting in AKT activation via mTORC2. Furthermore, mTORC1 inhibits PI3K signaling by adversely regulating growth factor receptor-bound protein 10 (Grb10). Rapamycin was so released Grb10 inhibition by mTORC1, leading to PI3K pathway stimulation of specific cell types (Figure 4).
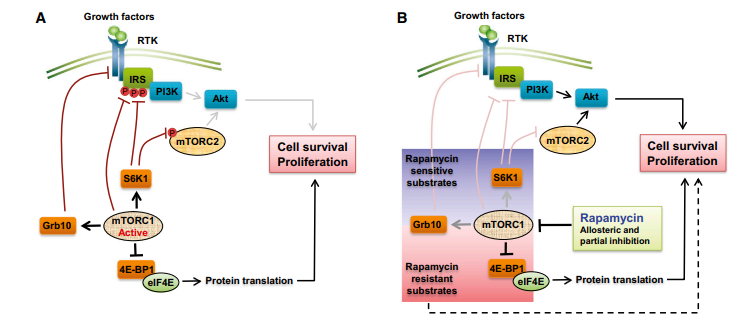
Figure 4: Many negative feedback loop in mTOR siginaling: A: When mTORC1 is activated, the RTK-PI3K signaling pathway is inhibited, which reduces Akt activation. B: Rapamycin inhibits negative feedback loops and enhances PI3K signaling activation.
Rapamycin has lot of potential, the recent advent of combination therapy with rapamycin may boost effectiveness even more and avoid the activation of survival pathways through feedback. There is a strong chance that efforts centered on investigating innovative drug combinations at ideal dosages will enhance safety and efficacy characteristics. Furthermore, there is still great potential for the identification of novel pathway inhibitors and the potential for currently available bioactives to either directly or indirectly lower the activity of TORC1 and/or TORC2 in monotherapy or in combination.













Comments