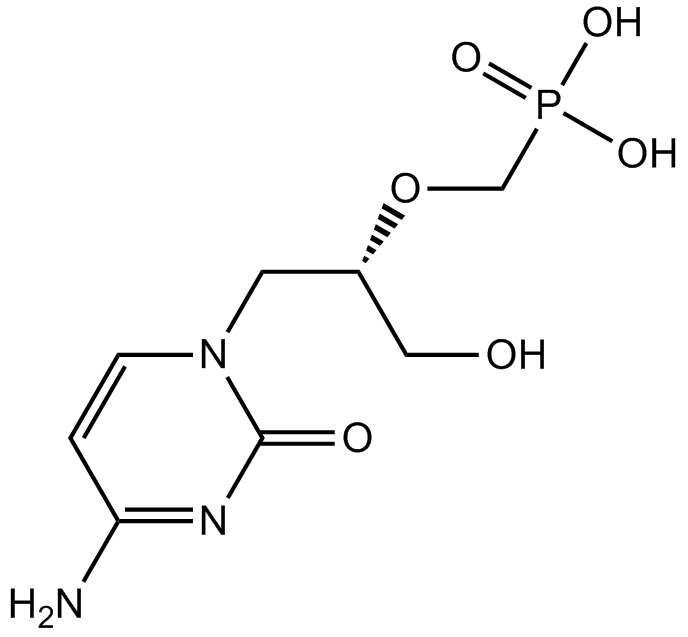
Cidofovir (Synonyms: GS-0504, (S)-HPMPC, (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine) |
| Catalog No.GC12666 |
시도포비르(GS 0504)는 비고리형 모노포스페이트 뉴클레오티드 유사체이자 항바이러스 활성이 있는 CMV 억제제입니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 113852-37-2
Sample solution is provided at 25 µL, 10mM.
Cidofovir is an anti-CMV drug which can suppress CMV replication by selective inhibition of viral DNA polymerase and therefore prevention of viral replication and transcription.IC50 Value:Target: CMV DNA polymerasein vitro: The minimum concentrations of (S)-HPMPC required to inhibit CMV plaque formation by 50% was microgram/ml. The selectivity indices of (S)-HPMPC, as determined by the ratio of the 50% inhibitory concentration for cell growth to the 50% inhibitory concentration for plaque formation for CMV (AD-169 strain), was 1,500 [1]. The time course of uptake of HPMPC into Vero cells was linear between 10 and 75 min and proportional to the concentration in the medium from 10(-6) to 10(-2) M. HPMPC uptake was temperature sensitive and the rate of uptake was considerably lower at 27 degrees than at 37 degrees and almost totally inhibited at 4 degrees [2]. in vivo: Levels of cidofovirin serum following intravenous infusion were dose proportional over the dose range of 1.0 to 10.0 mg/kg of body weight and declined biexponentially with an overall mean +/- standard deviation terminal half-life of 2.6 +/- 1.2 h (n = 25). Approximately 90% of the intravenous dose was recovered unchanged in the urine in 24 h. The overall mean +/- standard deviation total clearance of the drug from serum (148 +/- 25 ml/h/kg; n = 25) approximated renal clearance (129 +/- 42 ml/h/kg; n = 25), which was significantly higher (P < 0.001) than the baseline creatinine clearance in the same patients (83 +/- 21 ml/h/kg; n = 12) [3]. Positive CMV urine cultures reverted to negative in 2 of 8 patients receiving doses of < or = 1.5 mg/kg twice weekly and 11 of 13 patients receiving higher doses. Cidofovir has in vivo anti-CMV activity demonstrated by prolonged clearing of CMV viruria, although this observation is tempered by the fact that clearance of viremia could not be demonstrated [4].Toxicity: Patients receiving 0.5 or 1.5 mg/kg twice weekly experienced no serious toxicity. The first two patients who received 5 mg/kg twice weekly developed glycosuria and 2+ proteinuria. Subsequent patients received concomitant probenecid to attempt to ameliorate renal toxicity [4].Clinical trial: FDA approved drug
References:
[1]. Snoeck R, et al. (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 1988 Dec;32(12):1839-44.
[2]. Connelly MC, et al. Mechanism of uptake of the phosphonate analog (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in Vero cells. Biochem Pharmacol. 1993 Sep 14;46(6):1053-7.
[3]. Cundy KC, et al. Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1995 Jun;39(6):1247-52.
[4]. Polis MA, et al. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1995 Apr;39(4):882-6.
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















