Savolitinib (Synonyms: AZD6094, HMPL-504, Volitinib) |
| Catalog No.GC19321 |
사볼리티닙(AZD-6094)은 c-Met 및 p-Met에 대해 각각 IC50s가 5nM 및 3nM인 강력하고 고도로 선택적이고 경구 생체이용 가능한 c-Met 억제제입니다. Savolitinib(AZD-6094)는 ATP 경쟁적 방식으로 c-Met에 선택적으로 결합하여 활성화를 억제하고 c-Met 신호 전달 경로를 방해합니다. 항종양 활성.
Products are for research use only. Not for human use. We do not sell to patients.
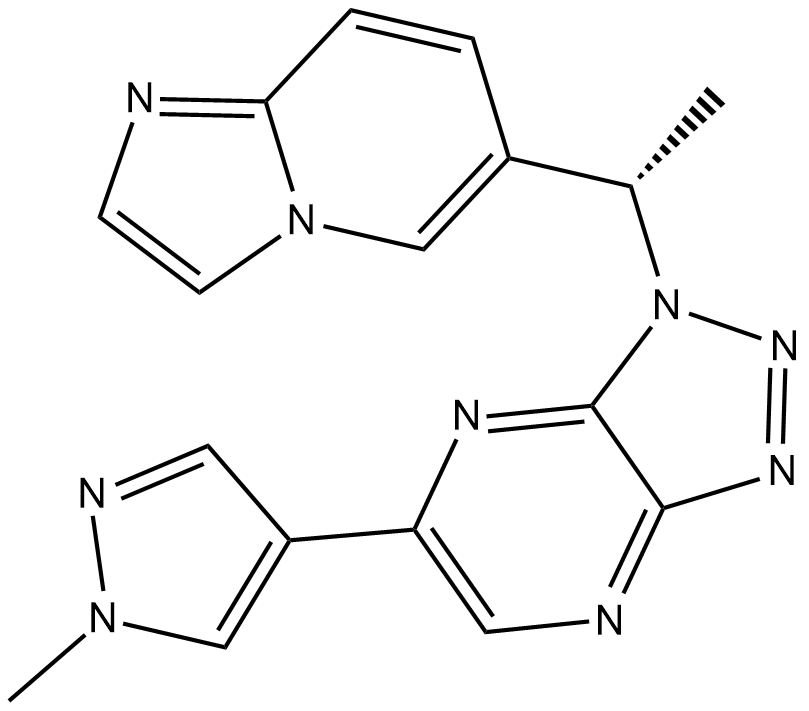
Cas No.: 1313725-88-0
Sample solution is provided at 25 µL, 10mM.
Savolitinib (AZD6094) ia highly potent and selective c-Met inhibitor with an IC50 of 5 nM.
References:
[1]. Jia H, et al. Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J Med Chem. 2014 Sep 25;57(18):7577-89.
[2]. Schuller A, et al. The MET inhibitor AZD6094 (Savolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient derived xenograft models. Clin Cancer Res. 2015 Mar 16. pii: clincanres.2685.2014.
[3]. Noh CK, et al. Simultaneous quantification of volitinib and gefitinib in rat plasma by HPLC-MS/MS for application to a pharmacokinetic study in rats. J Sep Sci. 2017 Jul 27.
Average Rating: 5 (Based on Reviews and 1 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















