Ziprasidone hydrochloride monohydrate (Synonyms: CP-88,059) |
| Catalog No.GC13627 |
An atypical antipsychotic
Products are for research use only. Not for human use. We do not sell to patients.
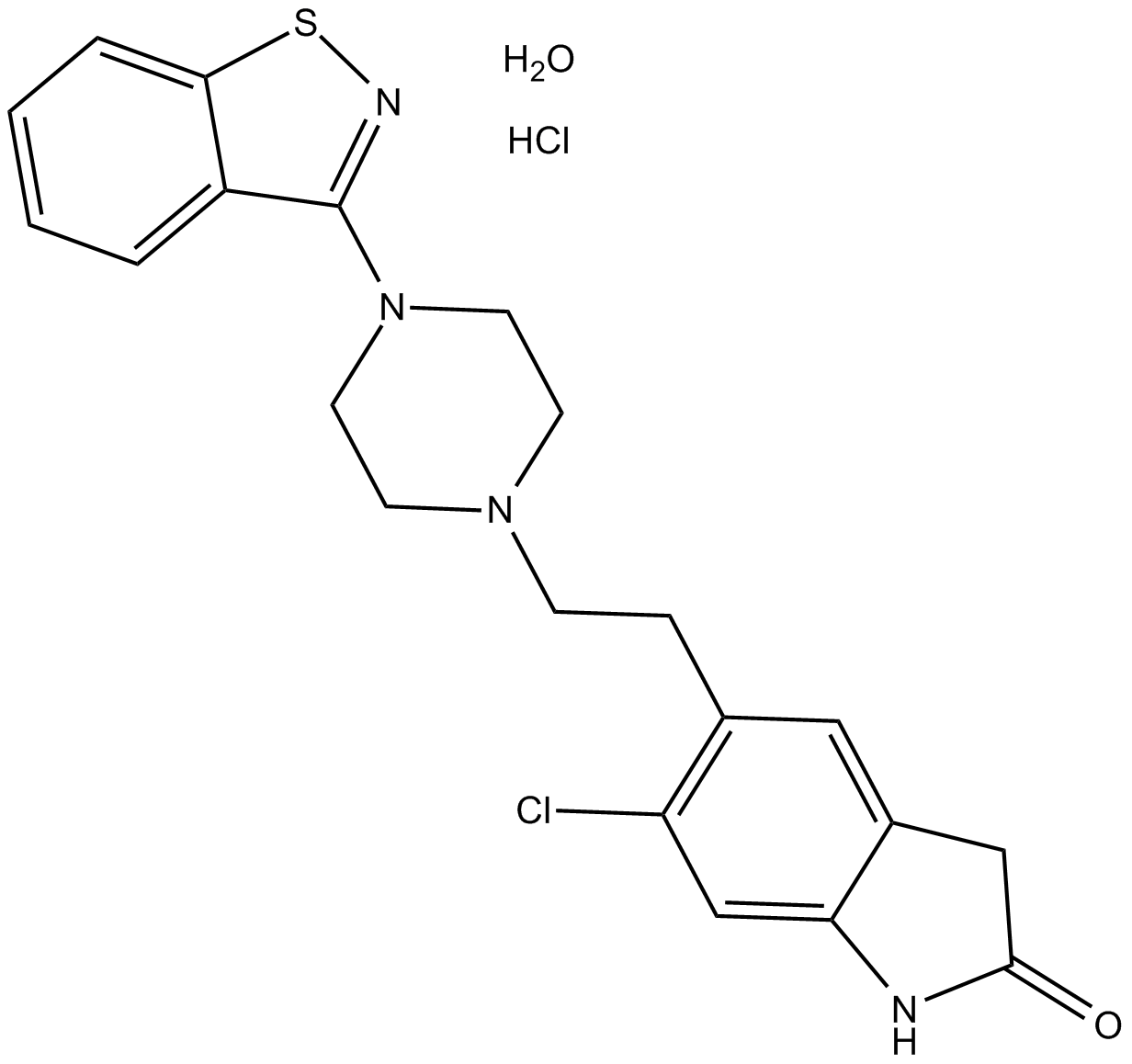
Cas No.: 138982-67-9
Sample solution is provided at 25 µL, 10mM.
Ziprasidone hydrochloride monohydrate (CP 88059 hydrochloride monohydrate) is a combined 5-HT (serotonin) and dopamine receptor antagonist which exhibits potent effects of antipsychotic activity.Target: 5-HT receptor; Dopamine receptorZiprasidone hydrochloride monohydrate is the salt form of ziprasidone, which possesses an in vitro 5-HT2A/dopamine D2 receptor affinity ratio higher than any clinically available antipsychotic agent. In vivo, Ziprasidone hydrochloride monohydrate antagonizes 5-HT2A receptor-induced head twitch with 6-fold higher potency than for blockade of d-amphetamine-induced hyperactivity, a measure of central dopamine D2 receptor antagonism. Ziprasidone hydrochloride monohydrate also has high affinity for the 5-HT1A, 5-HT1D and 5-HT2C receptor subtypes, which may further enhance its therapeutic potential [1]. Ziprasidone hydrochloride monohydrate sulfoxide and sulfone were the major metabolites in human serum. The affinities of the sulfoxide and sulfone metabolites for 5-HT2 and D2 receptors are low with respect to Ziprasidone hydrochloride monohydrate, and are thus unlikely to contribute to its antipsychotic effects [2]. Ziprasidone hydrochloride monohydrate was associated with significant differential adverse effects relative to placebo in BPM, BPD, and schizophrenia with no significant difference in weight gain in all 3 groups. Self-reported somnolence was increased across the 3 conditions. Subjects with BPM were more vulnerable to EPS than those with BPD or schizophrenia [3].Clinical indications: Bipolar I disorder; Bipolar disorder; Mania; SchizophreniaFDA Approved Date: February 2001
References:
[1]. Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. The Journal of Clinical Psychiatry. 2004, 65(2):217-221.
[2]. Paul E. Keck, Marcio Versiani, Steven Potkin, et al. Ziprasidone in the Treatment of Acute Bipolar Mania: A Three-Week, Placebo-Controlled, Double-Blind, Randomized Trial. Am J Psychiatry 2003;160:741-748.
[3]. Glen L. Stimmel, Mary A. Gutierrez, Vivian Lee. Ziprasidone: An atypical antipsychotic drug for the treatment of schizophrenia. Clinical Therapeutics, 2002, 24(1):21-37.
[4]. Anne W Schmidt, Lorraine A Lebel, Harry R Howard Jr. et al. Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. European Journal of Pharmacology. 2001,425(3):197-201.
[5]. Ziprasidone
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















