AEBSF.HCl (Synonyms: Pefabloc SC) |
| Katalog-Nr.GC14502 |
AEBSF.HCl is a broad-spectrum irreversible inhibitor of serine proteases, which can inhibit chymotrypsin, kallikrein, plasmin, thrombin, trypsin and related thrombolytic enzymes.
Products are for research use only. Not for human use. We do not sell to patients.
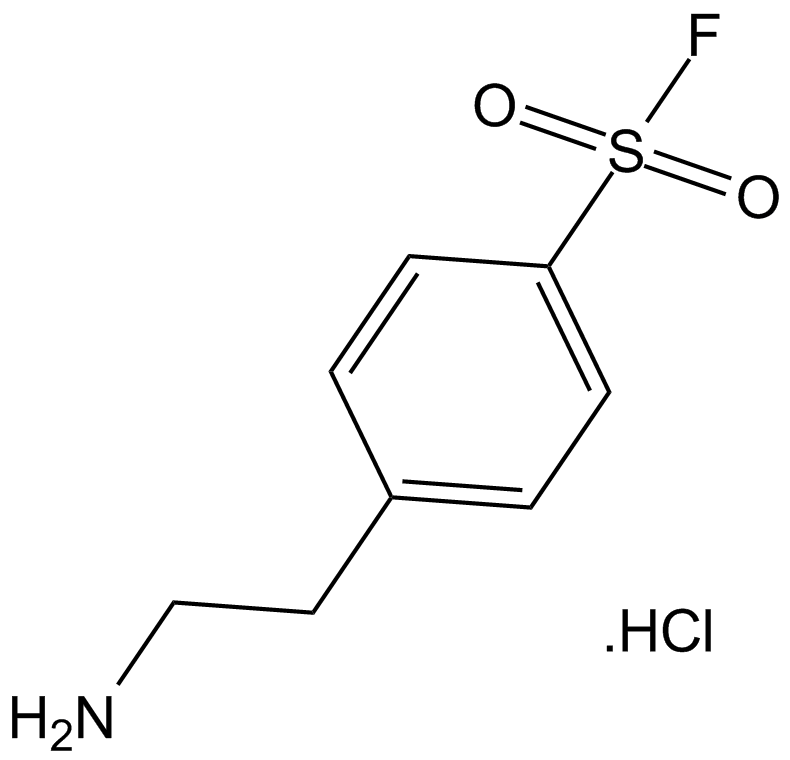
Cas No.: 30827-99-7
Sample solution is provided at 25 µL, 10mM.
AEBSF.HCl is a broad-spectrum, irreversible inhibitor of serine proteases, capable of inhibiting trypsin, kallikrein, plasmin, thrombin, chymotrypsin, and related thrombolytic enzymes. The mechanism of action of AEBSF.HCl involves covalent binding to serine residues, blocking the active center of the protease, thereby inhibiting its activity [1].
In vitro, AEBSF.HCl inhibits the production of Aβ in various cell lines. AEBSF.HCl demonstrates a dose-dependent reduction of Aβ in K293 cells transfected with K695 sw, with an IC50 value of about 1 mM, and shows a dose-dependent inhibitory effect in HS 695 and SKN 695 cells transfected with wild-type APP 695, with an IC50 value of about 300 μM. AEBSF.HCl also increases α-cleavage while inhibiting β-cleavage [1]. Additionally, as a protease inhibitor, AEBSF.HCl (150 μM) incubated with macrophages for 6 hours can block the lysis of leukemia cells by monocyte-derived macrophages [2].
In vivo, AEBSF.HCl (5 mg or 10 mg; i.v.) inhibits rat embryo implantation, with a visible reduction in the number of implanted embryos on day 8 of pregnancy [3]. AEBSF.HCl (76.8 mg/kg/d, i.p.) extends the survival time of mice given a lethal infection of Toxoplasma gondii [4].
References:
[1] Citron M, Diehl T S, Capell A, et al. Inhibition of amyloid β-protein production in neural cells by the serine protease inhibitor AEBSF. Neuron, 1996, 17(1): 171-179.
[2] Nakabo Y, Pabst M J. Lysis of leukemic cells by human macrophages: inhibition by 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), a serine protease inhibitor. Journal of leukocyte biology, 1996, 60(3): 328-336.
[3] Jiang YH, Shi Y, He YP, Du J, Li RS, Shi HJ, Sun ZG, Wang J. Serine protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) inhibits the rat embryo implantation in vivo and interferes with cell adhesion in vitro. Contraception. 2011 Dec;84(6):642-8.
[4] Buitrago-Rey R, et al. Evaluation of two inhibitors of invasion: LY311727 [3-(3-acetamide-1-benzyl-2-ethyl-indolyl-5-oxy)propane phosphonic acid] and AEBSF [4-(2-aminoethyl)-benzenesulphonyl fluoride] in acute murine toxoplasmosis. J Antimicrob Chemother. 2002 May;49(5):871-4
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















