Arphamenine B (hemisulfate) |
| Katalog-Nr.GC16829 |
aminopeptidase B inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
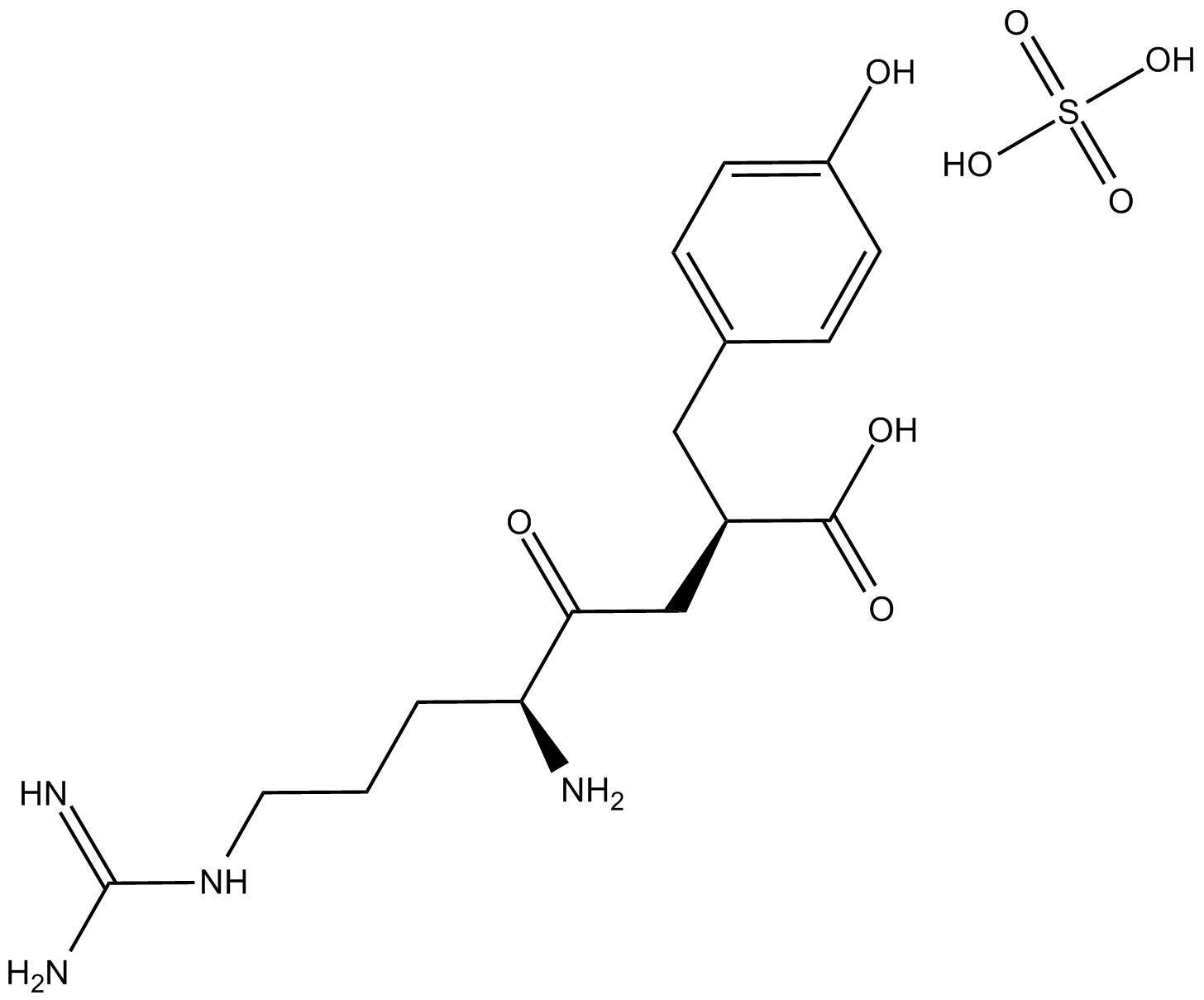
Cas No.: 144110-38-3
Sample solution is provided at 25 µL, 10mM.
Arphamenine B is a specific inhibitor of aminopeptidase B first isolated from bacteria [1]. Aminopeptidase B (Ap-B) is a Zn2+-dependent exopeptidase which selectively removes Arg and/or Lys residues from the N terminus of several peptide substrates. Aminopeptidase B has been involved in processing events occurring either during its intracellular transport along the secretory pathway or at the plasma membrane level [2].
In vitro: Arphamenine B inhibited the activity of aminopeptidase enzyme with an IC50 value of 9.0 μM [2]. Arphamenine B strongly inhibited transport by the oligopeptide/H+ symporter with the EC50 values of 15 to 67 μM. Arphamenine at concentration 100 μM acted as either ineffective or weak inhibitor of membrane-associated hydrolysis [4]. Arphamenine selectively suppressed dipeptide hydrolysis [4].
References:
[1] Umezawa H, AOYAGI T, OHUCHI S, et al. Arphamenines A and B, new inhibitors of aminopeptidase B, produced by bacteria[J]. The Journal of antibiotics, 1983, 36(11): 1572-1575.
[2] Balogh A, Cadel S, Foulon T, et al. Aminopeptidase B: a processing enzyme secreted and associated with the plasma membrane of rat pheochromocytoma (PC12) cells[J]. Journal of Cell Science, 1998, 111(2): 161-169.
[3] Sajid M, Isaac R E, Harrow I D. Purification and properties of a membrane aminopeptidase from Ascaris suum muscle that degrades neuropeptides AF1 and AF2[J]. Molecular and biochemical parasitology, 1997, 89(2): 225-234.
[4] Daniel H, Adibi S A. Functional separation of dipeptide transport and hydrolysis in kidney brush border membrane vesicles[J]. The FASEB journal, 1994, 8(10): 753-759.
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















