Coenzyme FO |
| Katalog-Nr.GC60720 |
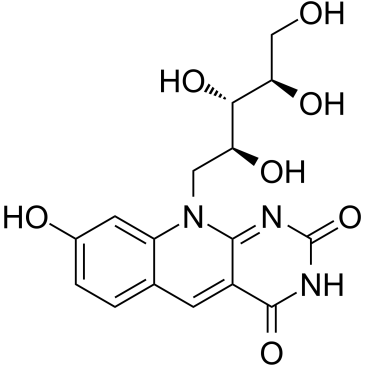
Das Coenzym FO, ein Deazaflavin-Chromophor, fungiert als wichtiger Hydrid-Akzeptor/Donator im zentralen methanogenen Stoffwechselweg.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 37333-48-5
Sample solution is provided at 25 µL, 10mM.
Coenzyme FO, a deazaflavin chromophore, acts as an important hydride acceptor/donor in the central methanogenic pathway[1][2].
The formation of Coenzyme FO is mediated by two separate radical SAM active sites, one each in the CofG and CofH enzymes or both in the FbiC enzyme. These two radical SAM domains constitute the functional domains of Fo synthase. F420 is biosynthesized in Methanocaldococcus jannaschii by the action of eight enzymes with the formation of the deazaflavin chromophore (Fo) as the remaining unsolved step[1].Coenzyme F420 is the central low-redox-potential electron carrier in methanogenic metabolism. Coenzyme F420 is reduced under hydrogen by the action of F420-dependent hydrogenase[3].Coenzyme F420 acts as a hydride transfer coenzyme for an F420-specific glucose-6-phosphate dehydrogenase (Fgd) in mycobacteria. Coenzyme F420 is found in all methanogenic and certain nonmethanogenic archaea, where it participates in energy metabolism, NADP reduction, oxygen detoxification, and sulfite reduction. By converting NO2 back to NO with F420H2, M. tuberculosis could decrease the effectiveness of antibacterial action of macrophages[4].
[1]. Mills DJ, et al. De novo modeling of the F(420)-reducing [NiFe]-hydrogenase from a methanogenic archaeon by cryo-electron microscopy. Elife. 2013;2:e00218. Published 2013 Mar 5. [2]. Philmus B, et al. Biosynthetic versatility and coordinated action of 5'-deoxyadenosyl radicals in deazaflavin biosynthesis. J Am Chem Soc. 2015;137(16):5406-5413. [3]. de Poorter LMI, et al. Hydrogen concentrations in methane-forming cells probed by the ratios of reduced and oxidized coenzyme F420. Microbiology. 2005;151(Pt 5):1697‐1705. [4]. Purwantini E, et al. Conversion of NO2 to NO by reduced coenzyme F420 protects mycobacteria from nitrosative damage. Proc Natl Acad Sci U S A. 2009;106(15):6333‐6338.
Average Rating: 5 (Based on Reviews and 37 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















