R-Palmitoyl-(1-methyl) Ethanolamide (Synonyms: R-1 PMA) |
| Katalog-Nr.GC13190 |
synthetic analog of palmitoyl ethanolamide (PEA)
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 142128-47-0
Sample solution is provided at 25 µL, 10mM.
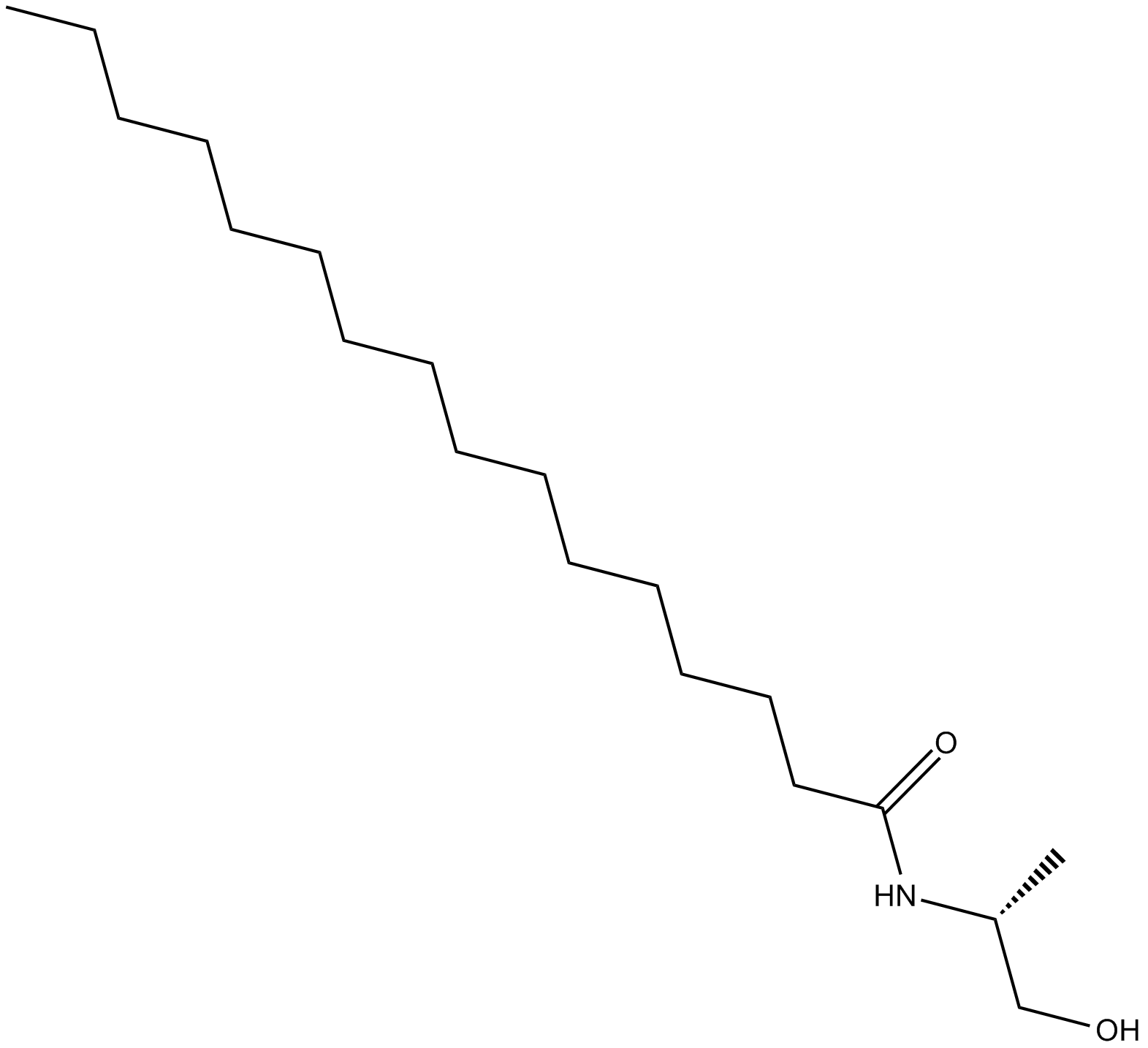
R-Palmitoyl-(1-methyl) ethanolamide, a synthetic analog of Palmitoyl ethanolamide (PEA), incorporates the (R)-methyl group vicinal to the alcohol on the ethanolamine moiety. The analogous modification to arachidonoyl ethanolamide (AEA) protects the molecule from hydrolysis by fatty acid amide hydrolase, prolongs duration of action and enhances potency in vivo [1].
PEA is an endogenous cannabinoid existed in mammalian tissues, such as liver and brain. PEA has also been isolated from egg yolk [2, 3]. PEA is a compound with documented anti-nociceptive and anti-inflammatory effects. During inflammation, PEA is accumulated and exihibits anti-inflammatory effects, including beneficial effects in clinically relevant animal models of inflammatory pain [4].
References:
[1] Abadji V, Lin S, Taha G, et al. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability[J]. Journal of medicinal chemistry, 1994, 37(12): 1889-1893.
[2] Bachur N R, Masek K, Melmon K L, et al. Fatty acid amides of ethanol-amine in mammalian tissues[J]. Journal of Biological Chemistry, 1965, 240: 1019-1024.
[3] Ganley O H, Graessle O E, Robinson H J, et al. Anti-inflammatory activity of compounds obtained from egg yolk, peanut oil, and soybean lecithin[J]. Journal of Laboratory and Clinical Medicine, 1958, 51: 709-714.
[4] Lambert D M, Vandevoorde S, Jonsson K O, et al. The palmitoylethanolamide family: a new class of anti-inflammatory agents [J]. Current medicinal chemistry, 2002, 9(6): 663-674.
Average Rating: 5 (Based on Reviews and 31 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















