5-Ph-IAA
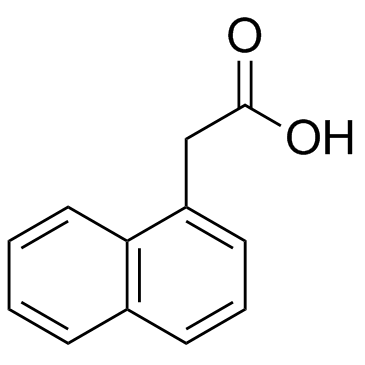
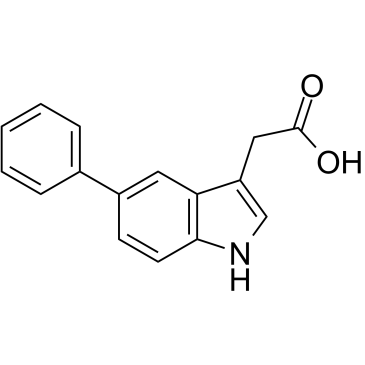
5-Phenyl-1H-indole-3-acetic acid is the full chemical name of the 5-Ph-IAA. Chemically, 5-Ph-IAA is a derivative of auxin/ indole acetic acid which is a plant hormone. 5-Ph-IAA is a synthetic, novel, small molecule, i.e., ligand, highly specific in its action. The two-dimensional view of the molecular structure of 5-Ph-IAA is presented in Figure 1.
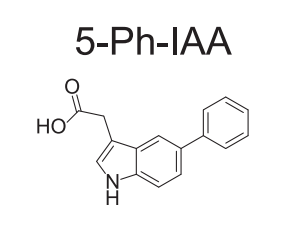
Figure 1 The two-dimensional view of the molecular structure of 5-Ph-IAA
5-Ph-IAA acts as a ligand in the Auxin-Inducible Degron 2 (AID2) system, which is the improved version of the Auxin-Inducible Degron (AID) system. Both AID/ AID2 systems were developed to discovery the functionalities of the protein molecules, which are the sequences of amino acids coded by the genes, within the alive cells via the application of protein degradation like many of the technologies that are available to reveal the protein functionalities. The former one, the AID system, was prone to unspecific protein degradation that is called as leaky degradation; therefore, the cells start dying. Moreover, in this technique, high concentration of auxins was needed. Combating all of these limitations, an improved version of the Auxin-Inducible Degron (AID) system was proposed, that is named as the Auxin-Inducible Degron 2 (AID2) system.
Previously, the Auxin-Inducible Degron (AID) system was the only way to study the protein functionalities. In the Auxin-Inducible Degron (AID) system, auxin, TIR1 are involved instead of 5-Ph-IAA, OsTIR1 (F74G) mutant. The AID system is given with the help of a schematic in Figure 2. In the scientific literature, there are several studies that documented the whole process to develop the Auxin-Inducible Degron 2 (AID2) system with better performance and results.
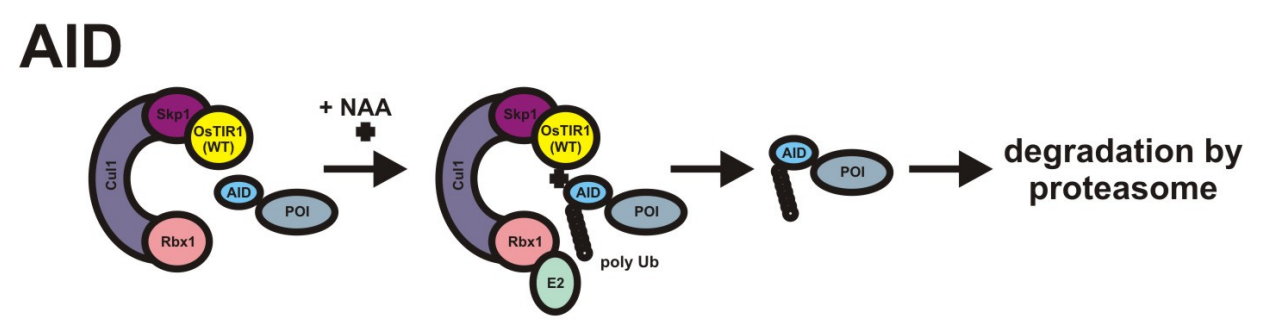
Figure 2 The molecular subunits involved in the AID System. The OsTIR1 in yellow, the AID tag in blue. As the ligand, NAA, is added, the target protein is poly-ubiquitinated and subsequently degraded by the proteosomes
As stated earlier, the core principle of Auxin-Inducible Degron 2 (AID2) system is the protein degradation. In this method, the protein of interest is only targeted and degraded using three important molecular subunits namely 5-Ph-IAA, OsTIR1 (F74G) mutant and a mini-AID that stands for Auxin-Inducible Degron. mini-AID is a type of Degron is nothing but a small protein segment whose size is 7kD, and is derived from Arabidopsis IAA12 and acts like a tag/ signal sequence on the target protein. Those proteins having this tag are identified by the SCF-OsTIR1 (F74G) E3 ligase and subjected to poly-ubiquitination followed by the enzymatic degradation of those protein molecules via the action of proteosomes. This protein degradation is achieved through the ubiquitin-proteosome pathway. Another interesting feature of this system that highlights the importance of 5-Ph-IAA in the Auxin-Inducible Degron 2 (AID2) system is that the whole protein degradation is controlled by the presence of 5-Ph-IAA alone. The complete underlying mechanism of the Auxin-Inducible Degron 2 (AID2) system is shown with the help of schematic diagram in Figure 3. This system is highly efficient in the terms of chemicals being used for the protein degradation such that less than 1 microMolar concentrated solution of 5-Ph-IAA is actually needed, which is 670 times less than the concentration of auxins needed in Auxin-Inducible Degron (AID) system. Apart from its efficiency, this system is also equally effective in the eukaryotic cells, for example, yeast, mice and mammalian cells.
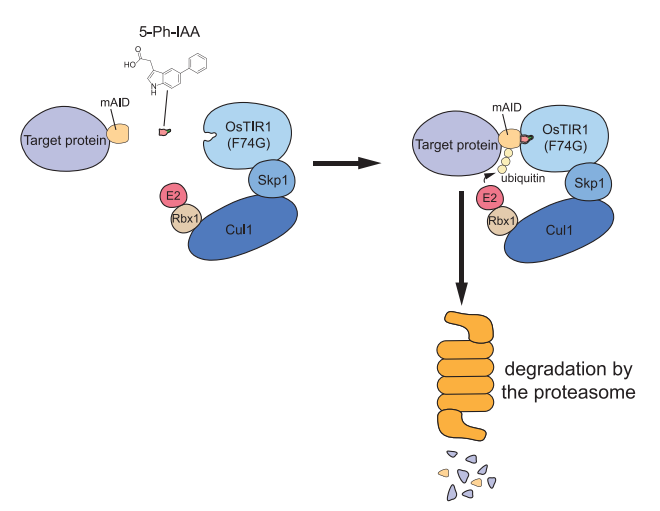
Figure 3 The core mechanism of the Auxin-Inducible Degron 2 (AID2) system via the ubiquitin-proteosome pathway. The target protein/ protein of interest would contain mini-AID tag. As soon as the 5-Ph-IAA ligand treatment is given to the target cell population, SCF E3 Ligase complex brings about the polyubiquitination of the target protein/ protein of interest; this step is followed by the biochemical degradation of the target protein/ protein of interest via the action of proteolytic enzymes of the proteosome.
5-Ad-IAA and 5-Ph-IAA, both are the analogous of the natural auxin. Both some comparable effectiveness in degrading the GFP-mIAA7. Their performance and effectiveness are far better than the NAA. This experimental observation shows the potential of 5-Ph-IAA in degrading wide range of targeted proteins. This observation is presented in Figure 4 with the help of a graph showing the GFP-mIAA7 degrading capabilities of the NAA, 5-Ad-IAA and 5-Ph-IAA.
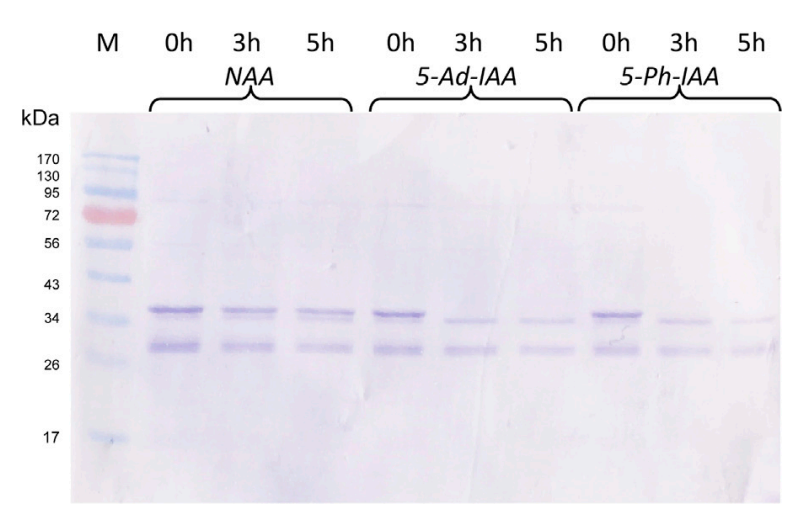
Figure 4 The GFP-mIAA7 degrading capabilities of the NAA, 5-Ad-IAA and 5-Ph-IAA. The protein depletion is directly proportional to the dimness of the protein bands. In the case of NAA, the protein bands are bright meaning less protein degradation. While, on the other hand, in case of 5-Ad-IAA and 5-Ph-IAA, almost same level of dimness is demonstrated by the protein bands indicating that most of the protein is degraded.
Revealing the protein functionalities within the living cells can be of significant interest in the field of molecular biology and cell biology. This is due to the fact that the multicellular organisms have cells having different protein profiles, some of the proteins are housekeeping which perform exactly the same function regardless of the cell in which they are expressed while there are other proteins as well that perform entirely different functions depending upon the cell type, the associated cellular organelle, the phase of the cell cycle, and the developmental stage. Therefore, the system such as Auxin-Inducible Degron 2 (AID2), can provide with a simple and rapid method to study this immense and diverse spectrum of protein functionalities while keeping the cell population still alive. This technique is specifically useful in studying the protein functionalities in the highly complex cellular processes like cell division and the cellular differentiation. Despite its actual real-world application in exploring the protein functionalities without any ambiguity, there are several drawbacks associated with this process which are explained below.
First, there are two processes in the development of the Auxin-Inducible Degron 2 (AID2) system where gene editing is a must requirement, i.e., for the engineering of the protein having mini-AID degron tag/ signal sequence, and for the engineering of Transport-Inhibitor Response 1 TIP1 which is F-box protein that binds the AID tag/ signal sequence. In a scientific research study, these two separate events were tried to be done at the same time by one transfection only. In that study, the gene for the target protein/ protein of interest is edited via the insertion of the sequence that have the mini-AID degron tag/ signal sequence as well as the sequence that codes for the Transport-Inhibitor Response 1 TIP1 protein; between these two lies a self-cleaving peptide sequence namely T2A and an internal ribosomal entry site namely IRES. The transcription of both of these added sequences was induced via the action of the same single CVM promotor sequence. After translation step of the protein expression, T2A cleaves the formed polypeptide such that the Transport-Inhibitor Response 1 TIP1 protein and the AID tagged target protein/ protein of interest get separated. The only limitation of this approach of inducing two gene editing steps as one step is that the AID tagged target protein/ protein of interest is expressed at a non-physiologic concentration. This limitation arises as the common promotor, i.e., CVM promoter is used.












コメント