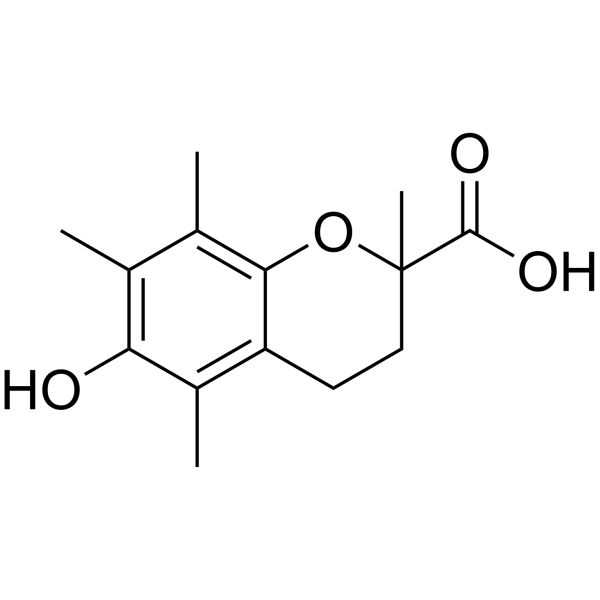
Forskolin
In the vast expanse of natural remedies and health supplements, there lies a potent compound that has been quietly making waves in the wellness community: Forskolin. Extracted from the Coleus forskohlii plant, a botanical relative of mint, this remarkable compound has transcended its traditional uses to become a beacon of hope for those seeking natural ways to improve their health. This article embarks on a journey to uncover the mysteries of Forskolin, exploring its origin, the science behind its benefits and how it can be integrated into your wellness routine.
Forskolin is not just a compound; it’s a testament to nature’s power to heal and enhance our well-being. It has been a staple in Ayurvedic medicine for centuries. Its recent surge in popularity is not without reason; modern science is beginning to validate what ancient practitioners have known all along.
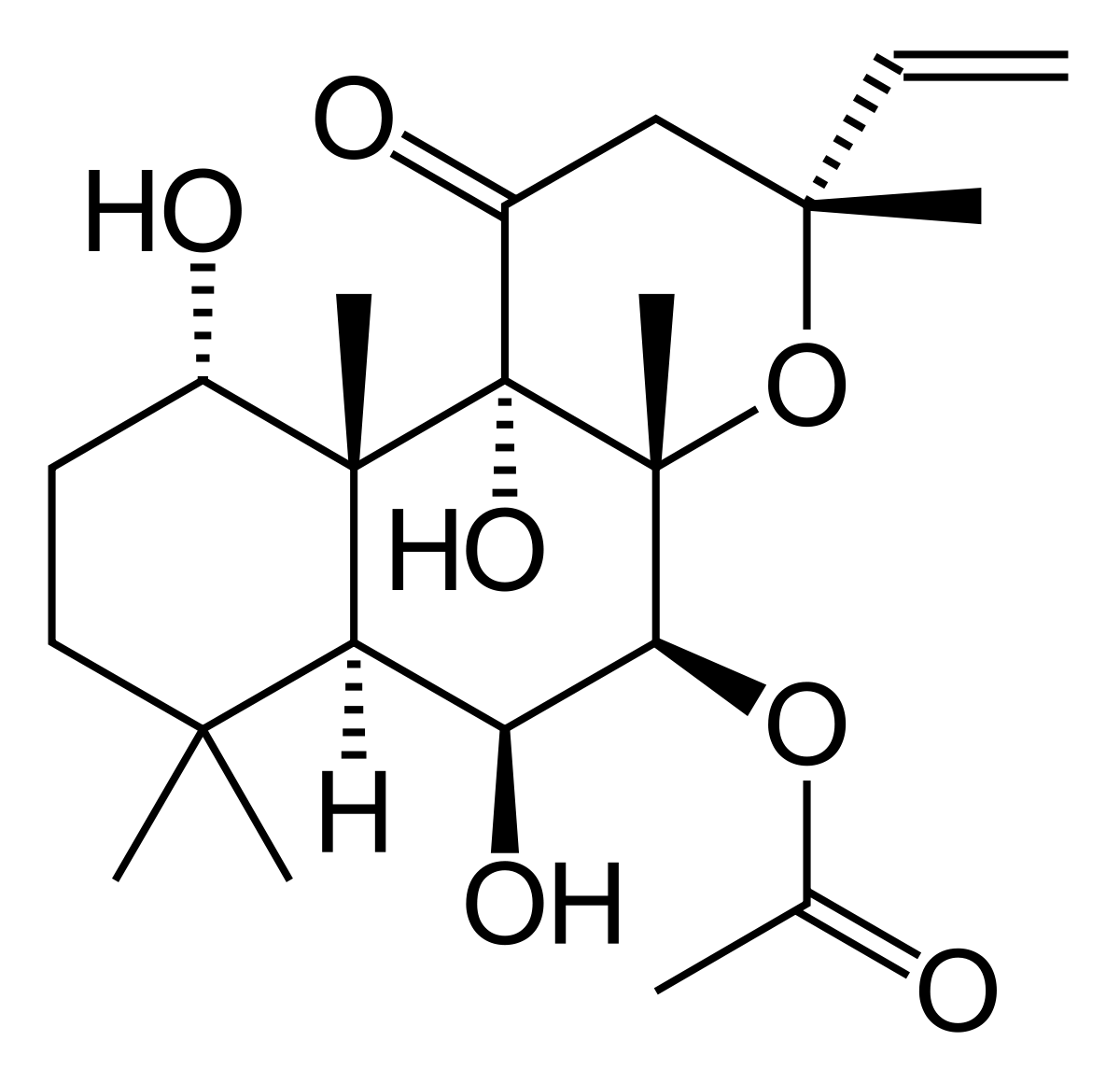
Forskolin is a unique structurally complex labdane-type diterpenoid used in the treatment of glaucoma and heart failure based on its activity as a cyclic AMP booster. Commercial production of forskolin relies exclusively on extraction from its only known natural source, the plant Coleus forskohlii, in which forskolin accumulates in the root cork. Here, we report the discovery of five cytochrome P450s and two acetyltransferases which catalyze a cascade of reactions converting the forskolin precursor 13R-manoyl oxide into forskolin and a diverse array of additional labdane-type diterpenoids. A minimal set of three P450s in combination with a single acetyl transferase was identified that catalyzes the conversion of 13R-manoyl oxide into forskolin as demonstrated by transient expression in Nicotiana benthamiana
Forskolin is a direct activator of adenylate cyclase enzyme and a largely known cAMP elevating compound. However, it can affect other cellular activities. To verify whether the forskolin could be effectively attributed to the cAMP increase we also evaluated the effects of another cAMP agent on the GSKJ4-induced cytotoxicity. To this purpose, we treated or not (control) U937 cells for 24 h with GSKJ4 10 μM in the absence or presence of 2 mM IBMX, a broad-spectrum phosphodiesterase inhibitor (Figures 3A,B). After treatments, direct cell number counting and PI uptake cell death assays were performed. In Figures 3A,B it is shown that the proliferation of U937 cells was not obviously affected by IBMX, when used alone. Interestingly, we found that the co-treatment with cAMP elevating agent IBMX enhanced the anti-proliferative effects of GSKJ4, at similar extent of forskolin (Figures 3A,B). In addition, we included in these experiments 8-Br-cAMP, a common analog of cAMP, and 8-pCPT-2′-O-Me-cAMP, a cAMP analog, which specifically activates Epac and not PKA Similarly to forskolin, 8-Br-cAMP, which is expected to activate both PKA and Epac, potentiated the GSKJ4-induced cytotoxicity. In contrast, the Epac activator 8-pCPT-2′-O-Me-cAMP had only a minimal impact on GSKJ4 effect. Overall, the above data indicate that forskolin potentiates the sensitivity of U937 cells to GSKJ4 via cAMP elevation and that, very likely, PKA might be involved in.
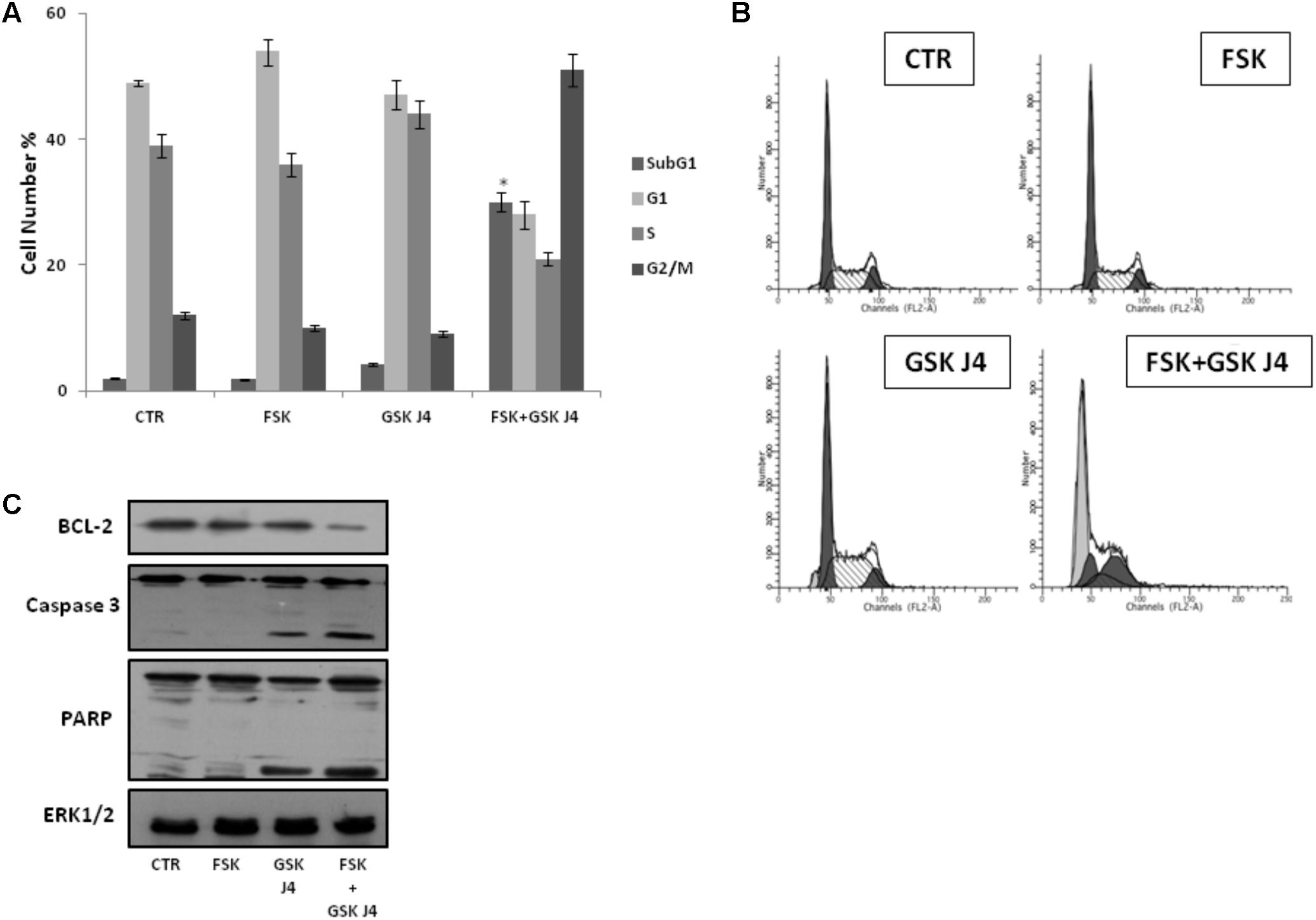
Figure 2: Forskolin Enhances the Sensitivity of U937 to GSKJ4 by Inducing Apoptotic Cell Death
Effects of forskolin, GSKJ4, and forskolin/GSKJ4 combination on the distribution of U937 cells in cell cycle and sub-G1 phases, and on some relevant apoptotic proteins. Treatments with 10 μM forskolin, 10 μM GSKJ4, and forskolin/GSKJ4 combination were carried out for 24 h. Subsequently, sub-G1 and Cell Cycle Phases (panels A, B) were evaluated by Flow Cytometry. In panel A, quantitative data from four independent experiments indicating the percentage of sub-G1, G1, S, and G2/M cells are shown. The means and SD are shown. ∗P < 0.05, compared to control untreated cells. In panel B, representative qualitative data of a typical experiment are shown. In panel C, the levels of the indicated proteins were assessed by western blotting from 20 μg of protein in total cell extract. The image shown is representative of three different experiments with similar results.
Cystic fibrosis is caused due to a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Two potential drug targets can be used to treat cystic fibrosis; namely, potentiator VX-770 and corrector VX-809 linked to CFTR gene. CRE sequence (TGACaTCA) present in the promoter CFTR gene has thrown more light on the processes from cAMP regulation to gene expression. Around 45–50% of cystic fibrosis patients suffer from a homozygous mutation named, F508DEL. In 1991, Drumm et al. reported that the association between CFTR and chloride conductance is sensitive to forskolin (Figure 3), where the order of sensitivity occurs at a similar level as the disease severity. Several clinical studies are reported for cystic fibrosis, such as NCT03652090, NCT03390985, NCT03894657 and NCT02807415, where forskolin is used to analyze drug sensitivity and classification of cystic fibrosis.
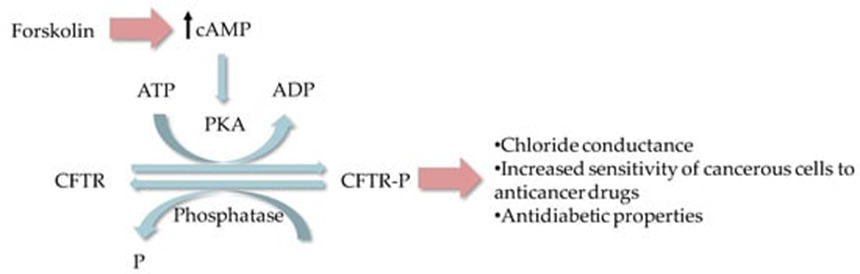
Figure 3. Potential modulation effect of forskolin based on [36]. ADP, adenosine diphosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; PKA, cAMP-dependent protein kinase; CFTR, cystic fibrosis transmembrane conductance regulator; CFTR-P, phosphorylated CFTR.
Forskolin is neuroprotective by the activation of MAP kinase(ERK1/2). We demonstrate here that the effect of NA is strongly enhanced by cAMP-elevating agents, in particular forskolin (FK), through a mechanism that does not involve activation of adrenoceptors. FK also enhanced the neuroprotective action of antioxidants that mimic the trophic effects of NA, such as trolox and pyrocatechol, but was totally ineffective by itself, suggesting that inhibition of oxidative stress was a required step to reveal the cAMP-dependent mechanism. Neuroprotection afforded by FK was rapidly reversible, optimal when the treatment was initiated in the early phase of the culture and exquisitely specific to dopaminergic neurons. FK stimulated the phosphorylation of extracellular signal-activated kinases (ERK)(1/2) in a subpopulation of dopaminergic neurons,suggesting that the mitogen-activated protein kinase (MAPK) pathway was involved in the effects of cAMP-elevating agents. Accordingly, inhibition of the upstream kinases of ERK(1/2) by 2'-amino-3'-methoxyflavone (PD98059) not only suppressed MAPK activation caused by FK but also abolished the survival promoting activity that this compound exerts on TH(+) neurons. PD98059 did not reduce, however, the trophic effects provided by NA alone. Surprisingly, the archetypal cAMP-dependent protein kinase was apparently not responsible for ERK(1/2) activation. The data suggest that the MAPK signaling pathway plays a key role in the trophic effects that cAMP elevating agents and NA cooperatively exert on TH(+) neurons.
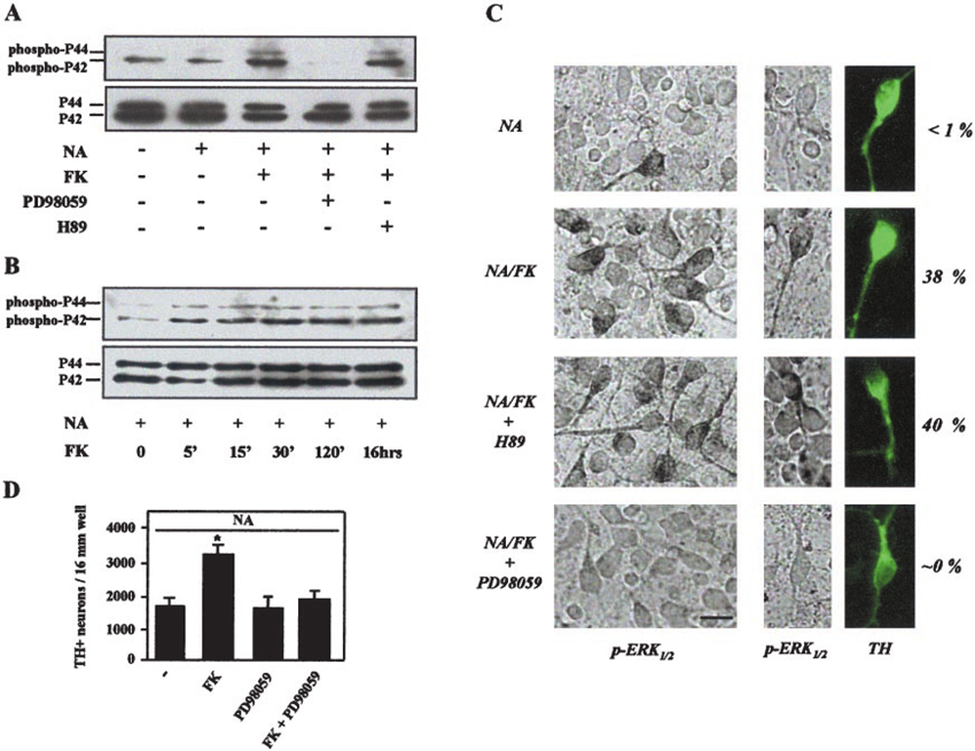
Figure 4: Forskolin is neuroprotective by the activation of MAP Kinase(ERK1/2).
Forskolin is neuroprotective by activation of the MAP kinase (ERK 1/2 ) signaling pathway. A, Western blotting analysis of pERK 1/2 expression in cultured DIV 1 mesencephalic cells exposed for 15 min to various test treatments. The MEK inhibitor PD98059, but not the PKA inhibitor H89, abolished FK-dependent increase of ERK 1/2 phosphorylation. Protein loading was controlled by additionally staining blots with a non–phospho-ERK 1/2 antibody. B, FK induction of ERK 1/2 phosphorylation was long-lasting. C, immunodetection of pERK 1/2 using the chromogen diaminobenzidine in DIV 1 cultures exposed for 15 min to NA alone or in the presence of FK, FKPD98059, or FKH89. Subsequent visualization of TH neurons by fluorescein isothiocyanate immunofluorescence showing that FK produces an increase in the number of dopaminergic neurons immunopositive for pERK 1/2 . Consistent with the results obtained by Western immunoblotting, PD98059 but not H89 prevented FK-induced ERK 1/2 phosphorylation at the cellular level. D, promotion of cell survival by FK in NA-treated cultures was suppressed by pharmacological inhibition of MEK using PD98059. PD98059 failed, however, to reduce the neuroprotective action of NA alone. NA, 1 M; FK, 25 M; PD98059, 10 M; H89, 1 M. , p >0.05, versus cultures exposed to NA alone.
Forskolin is a naturally derived diterpenoid that can interact with the cAMP pathway.
This interaction endows forskolin with significant therapeutic benefits against several metabolic diseases, cancers and others. According to a number of clinical studies reported on clinicaltrials.gov, the effects of forskolin have been studied in conditions such as asthma, cystic fibrosis, homozygous F508DEL mutation, chronic obstructive pulmonary disease (COPD), metabolic syndrome, obesity and glaucoma. Notable clinical and preclinical studies, along with their pharmacological actions, are discussed below. Moreover, a summary of the effects of forskolin is shown below:
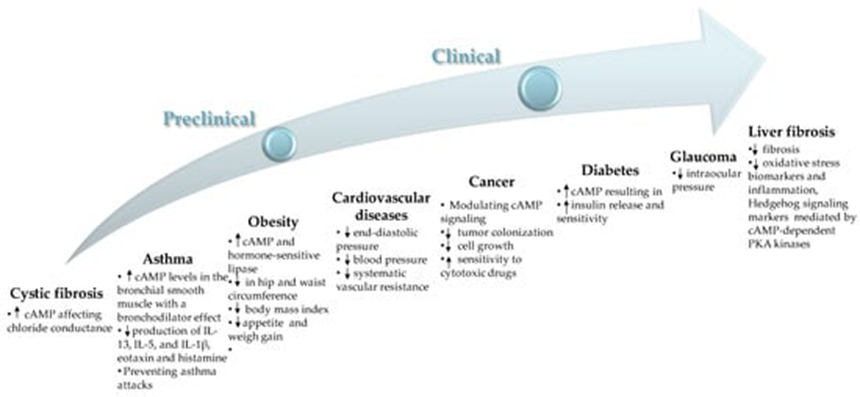
Forskolin presents an intriguing option for those looking to enhance their health through natural means. Its potential benefits range from weight loss and improved heart health to asthma relief and beyond. However, as with any supplement, it's crucial to use Forskolin responsibly and in conjunction with professional medical advice. As research continues to unfold, we may discover even more about the capabilities of this fascinating compound.












コメント