Blasticidin S HCL
Blasticidin S HCL being a nucleoside analogue antibiotic, exhibits broad-spectrum efficacy against eukaryotic and prokaryotic cells. Isolated from Streptomyces griseochromogenes. Blasticidin S serves as the selection agent while demonstrating many antiviral, anti-tumor, and antifungal properties way beyond its antimicrobial activity. It has been used as a selection agent to enrich cells harboring the bls, bsr, or BSD resistance gene, including HEK293-T and HEK-D5 cell lines with its extended effectiveness in investigating protein translation mechanisms and preventing culture contamination.
Unveiling Structural Features for Functional Understanding of Blasticidin S HCL:
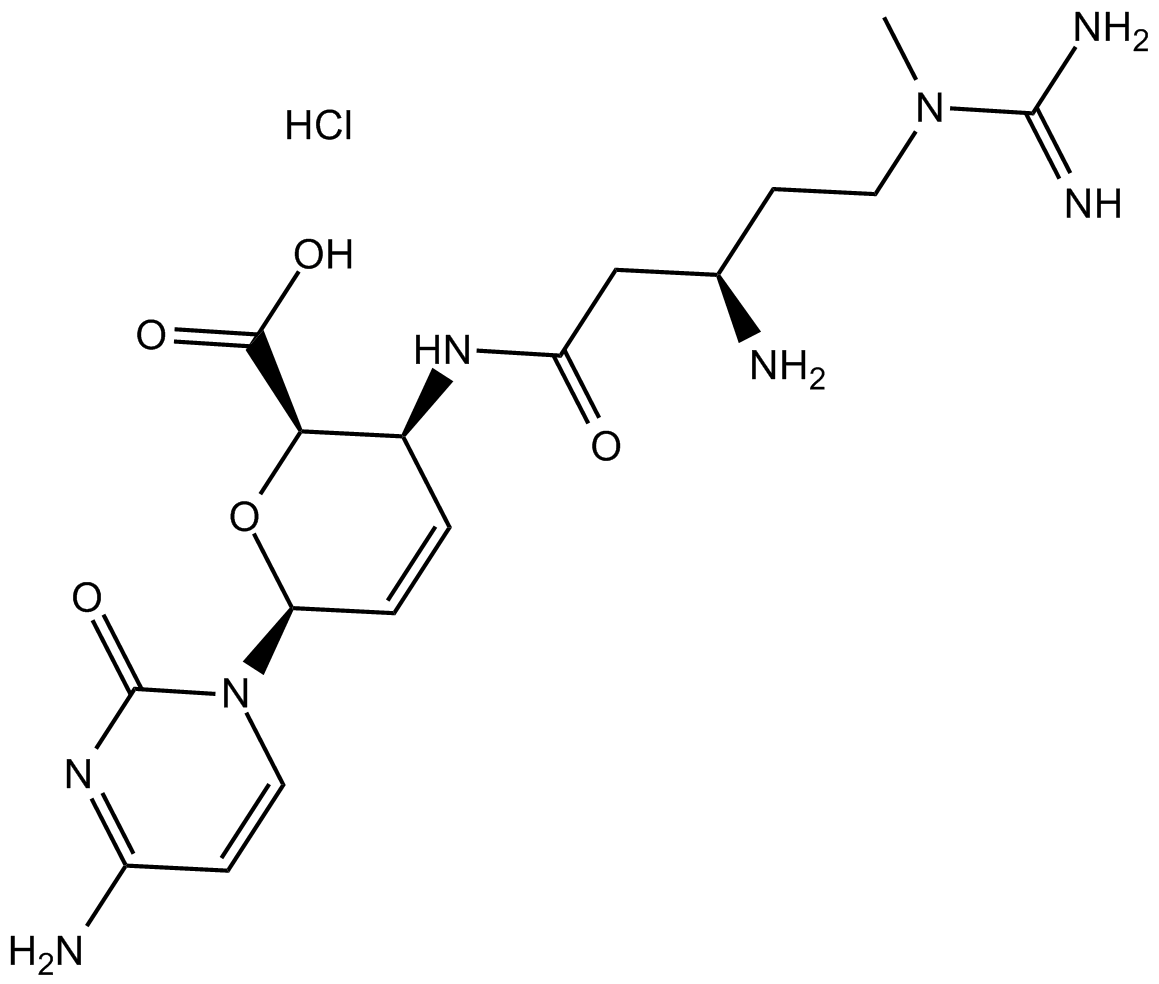
Blasticidin S HCL exhibits several key properties relevant to its scientific application as it is a nucleoside antibiotic with a molecular weight of 458.9 g/mol with the exact and monoisotopic masses (458.1792937 g/mol) that contributes to providing precise characterization for spectroscopic techniques. Blasticidin S HCL indicates a high potential for the formation of stable interactions with other molecules due to its property that it possesses a high hydrogen bond donor and acceptor count (7 each), with some degree of conformational flexibility and rotational freedom. The presence of 31 heavy atoms contributes to the overall molecular architecture with a defined atom stereocenter count of 4 and a topological polar surface area of (213 Ų) which potentially influences solubility and membrane permeability by reflecting a balance between hydrophilic and hydrophobic regions. A detailed picture of the molecular level of Blasticidin S HCL with the lack of undefined stereocenters ensuring a consistent and well-defined structure can provide the foundation for a deeper comprehending of its biological function and potential applications in the realm of scientific research. The Chemical Structure of Blasticidin S HCL comprises of Blasticidin S free base, a novel nucleoside moiety identified as cytosine (C10H12O4N4), a newly discovered amino acid blastidic acid(C7H16O2N4), Hence possessing a definitively characterized molecular formula, C17H26O5N8. Selective adsorption of antibiotic onto a cation exchange resin followed by elution with hydrochloric acid is a straightforward process through which the isolation and purification of the antibiotic can be achieved with the Subsequent concentration under vacuum yielding a crude hydrochloride salt.
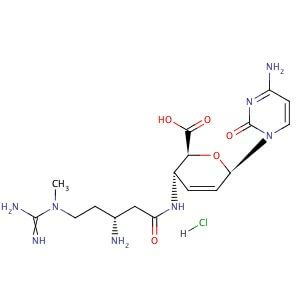
Fig 1: Structure of Blasticidin S HCL
Blasticidin S: From Agricultural Fungicide to Versatile Biological Tool :
The distinction of being the first fermentation-derived antibiotic is held by Blasticidin-S which was designed specifically for agricultural applications. Its remarkable fungicidal properties were elucidated in 1958, after its possible isolation from the culture broth of S. griseochromogenes in 1955. A diverse array of biological activities are exhibited by this (Blasticidine S) nucleoside analog encompassing antiviral, antimicrobial, and antineoplastic effects. Bisticidine agricultural significance has diminished due to its (phyto) toxic properties contributing as a limiting factor even when the Blasticidin application rates of 10–40 g/ha demonstrated excellent control of the rice fungal pathogen, Pyricularia oryzae. Inhibition of peptide bond formation at the ribosome is due to the Blasticidine S action exertion and is achieved by the hindration of the peptidyl-tRNA translocation step due to the tRNA affinity of the large ribosomal unit enhanced. ASTs are performed against both Gram-negative and Gram-positive bacteria using panels, discs, and minimum inhibitory concentration (MIC) strips by Medical microbiologists. The test results for AST facilitate clinicians in the selection of the most appropriate antibiotic(s) for the treatment of various infections.
Research insights into Blasticidin S Deaminase as a New Selectable Marker for Chlamydomonas reinhardtii : For both fundamental and applied research areas, including biofuel and high-value molecule production, Chlamydomonas reinhardtii is a well-established unicellular model organism due to its photosynthetic capabilities. A versatile Chlamydomonas Modular Cloning toolkit has been established, encompassing 119 biobricks to facilitate the development and optimization of various genetic constructs and strains. The ability to utilize a diverse range of selectable markers is a crucial aspect of forward and reverse genetics in Chlamydomonas. The development of a novel selectable marker based on resistance to the antibiotic blasticidin S, achieved through the incorporation of the Bacillus cereus blasticidin S deaminase (BSR) gene was described in this mentioned research study. For efficient selection of the optimal blasticidin S concentration in both liquid and solid media was determined and validated across several laboratory strains. The newly developed selectable marker does not compromise the efficacy of other commonly used antibiotic resistances: zeocin, hygromycin, kanamycin, paromomycin, and spectinomycin as demonstrated by the compatibility testing. Integration of blasticidin resistance biobrick into the Chlamydomonas is done through Modular Cloning toolkit making it readily available to the scientific community.
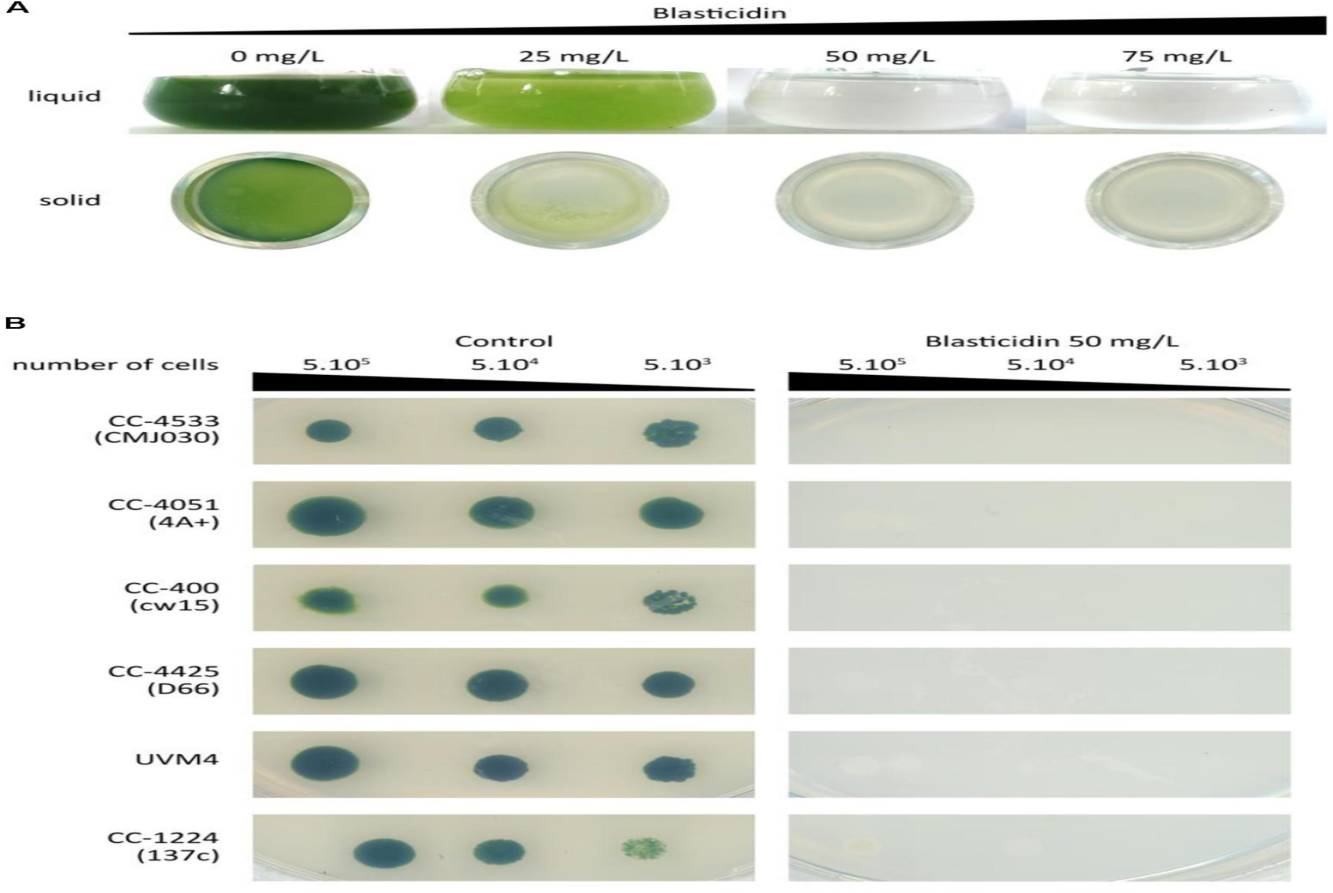
Fig 2 : Chlamydomonas strains showed sensitivity to blasticidin in a dose-dependent manner. Growth inhibition was observed at increasing concentrations (25, 50, and 75 mg/L) of blasticidin in both liquid and solid media. Similarly, spot tests confirmed sensitivity to blasticidin (50 mg/L) for six standard laboratory strains.
Blasticidin S Resistance Gene: A Boon for B. subtilis Manipulation and Unveiling Mammalian Ribosome Inhibition Mechanism: Originally identified in Bacillus cereus, Blasticidin S resistance gene (bsr) has been investigated for its functionality in Bacillus subtilis as this could be a valuable tool for the genetic manipulation studies in B. subtilis and the development of novel selection marker B. subtilis. A DNA fragment comprising of the entire bsr gene with encompassed 617 base pairs (bp) along with upstream 5' flanking region has been discovered to confer resistance to Blasticidin S (BS) in B. subtilis where a single copy of this DNA fragment is sufficient enough to bestow BS resistance into the B. subtilis chromosome. By employing a multifaceted approach, integrating biochemical, structural, and cellular techniques, Blasticidin S is targeted binding to the peptidyl transferase center of the large ribosomal subunit in mammals in both elongation and termination stages of translation and exerting its ribosome-inhibitory effects. Compared to what was observed in bacteria, Blasticidin distorted the P-site tRNA's 3'CCA terminus contributing to the delaying of both by binding to the peptide bond formation and peptidyl-tRNA hydrolysis by binding to the mammalian terminating ribosomes. Intriguingly, Blasticidin obstructs subsequent peptide release by hindering eRF1's proper accommodation within the peptidyl transferase center and not interfering with stop codon recognition by eukaryotic release factor 1 (eRF1). Therefore, Blasticidin leads to the enhancement in protein production at subinhibitory concentrations by modulating the translational dynamics at premature termination codons, exhibiting the ability to suppress nonsense-mediated mRNA decay in human cells.
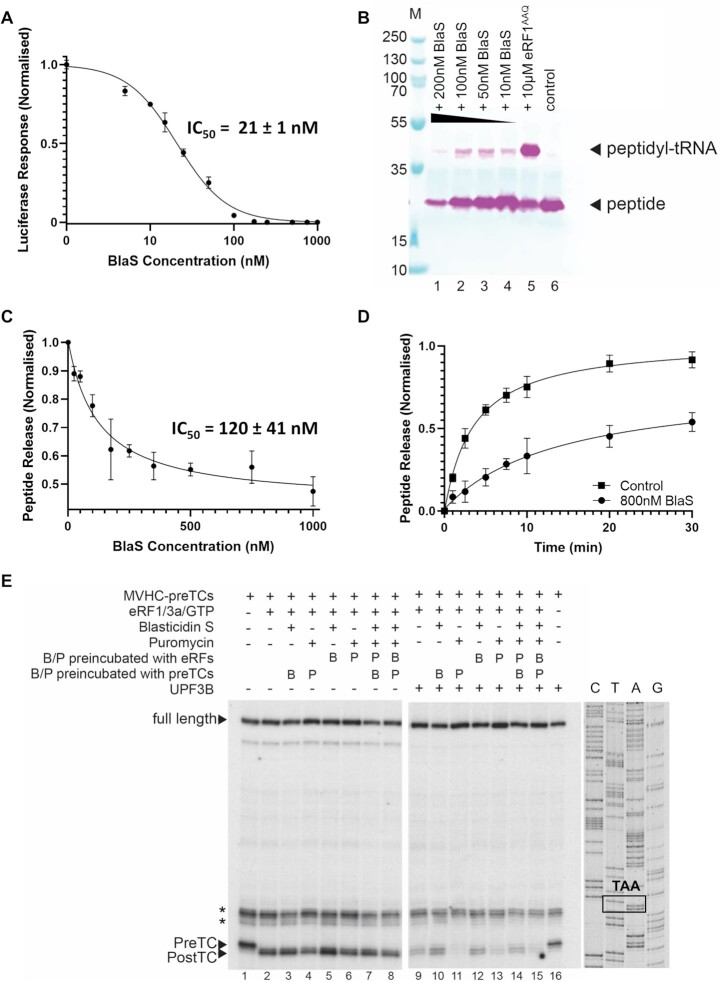
Fig 3: demonstrates the inhibitory effect of Blasticidin in mammalian translation
Blasticidin S: Unveiling its Role in Modern Cell Biology: The stable and non-phytotoxic BABS salt is the commercially available formulation of Blasticidin S which is offered in various formats including dispersible powders, emulsifiable concentrates, or wettable powders, with active ingredient concentrations ranging from 1.4–6%. An improved formulation incorporating 5% calcium acetate was introduced to mitigate ocular irritation. Blasticidin S is finding extensive use in laboratory settings for the selection of transfected cells harboring resistance genes, beyond its use in agriculture as these genes encode an enzyme, acetyltransferase or deaminase enzymes, playing a critical role in the detoxification of the antibiotic.
Cautious Handling: Classified under the Globally Harmonized System (GHS) with a Danger signal word, Blasticidin S HCL presents a significant safety hazard exhibiting acute toxicity upon oral ingestion (H300) and dermal contact (H312) with warranting strict handling protocols. The 73.68% classification ratio for acute dermal toxicity (Acute Tox. 4) is preventing skin cancer by necessitating appropriate personal protective equipment (PPE), highlighting the critical importance to ensure safe handling and minimizing the risk of exposure with Blasticidin S HCL during scientific experimentation by adhering to the designated precautionary measures (P-codes).
In conclusion, Blasticidin S HCL stands as an antibiotic with a fascinating biosynthetic pathway and well-defined molecular structure with its remarkable inhibitory effects on translation even beyond the bacterial realm as the article also provided insights on its inhibitory effects in a mammalian cell. Blasticidin S HCL has the potential to serve as a,a valuable tool in paving the way for novel drug discovery efforts and scientific exploration in elucidating protein synthesis regulation.













コメント