Denosumab
Denosumab is novel pharmaceutical drug. Chemically, it is a monoclonal antibody (mAb), that has the full-length sequence same as of the human antibodies; thus, it is sometimes regarded as fully human. It was initially developed using XenoMouse transgenic mouse technology, which is a powerful tool used for the synthesis of human antibodies in bulk quantity. It selectively and actively binds to the Receptor activator of nuclear factor kappa-Β ligand (RANKL) with a strong interaction. It has a good safety profile as a therapeutical drug against osteoporosis. Other reason that makes Denosumab not only the suitable but also the best treatment option is that it has a longer half live. That essentially means that less frequency of the Denosumab dosage would be required. Less dosage frequency is an important factor when it comes to use any pharmaceutical drug for the therapeutic purposes in the clinical setting.
Denosumab is a clinically, i.e., more specifically saying therapeutically, approved and used as pharmaceutical drug to reduce the chances of bone fractures, both vertebral and non-vertebral ones. This is especially true for those bone fracture which are caused by osteoporosis in old women after menopause. It is also clinically used to treat the bone irregularities as in the Rheumatoid Arthritis (RA). in clinical settings, it is subcutaneously administrated.
Physiologically, it increases the Bone Mineral Density (BMD) as it increases the bone mass. Thus, acts as antiresorptive or anticatabolic in nature. This effect is of therapeutical significance against osteoporosis and well exploited in the clinical settings.
There exists a physiological balance between the bone formation and bone resorption; that’s how a definite healthy bone mass is maintained in the human body. Bone formation is mediated by the activity of osteoblasts, while the bone resorption is mediated by the activity of the osteoclasts. These two distinct cellular processes are linked via a molecular ligand, known as Receptor activator of nuclear factor kappa-Β ligand (RANKL) that is produced and released via the active osteoblast. These RANKLs binds to the RANK which is expressed on the surface of the osteoclasts, i.e., precursors and as well as the mature ones. Binding of RANKL with the RANK, activates the osteoclasts. Activated osteoclast produce and release such enzymes which brings about degradation of proteins, i.e., proteolytic enzymes which cause the breakdown of collagen. This leads to the formation of cavity in the bone and also a decrease in the bone mass. In short, we can say that active osteoblasts activate the osteoclasts, via releasing RANKL. As a take home message, it can be concluded that the activity of osteoclasts is dependent on the activity of the osteoblasts. If there is some bone formation, there is also bone resorption. This bone modeling process as a physiological balance, along with its core players are diagrammatically depicted in Figure 1 .
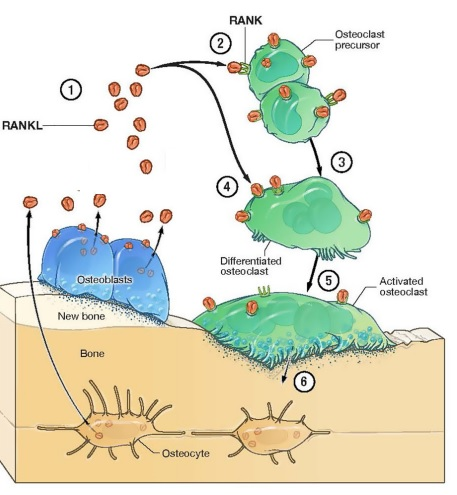
Figure 1 The main players in the bone remodeling, i.e., osteoblasts and osteoclasts. Bone Remodeling is a physiological process. The bone modelling process maintains the bone mass. It is essentially a balance between the activity of osteoblasts (as shown in blue) and osteoclasts (as shown in green). A key link and mediator between active osteoblasts and osteoclasts, i.e., RANKL (as shown in orange).
Denosumab interacts with the bone modeling process in a way that is different from the rest of the pharmaceutical drugs for the osteoporosis. In fact, denosumab eliminates this condition that if there is a bone formation, there is always a bone resorption; it does so by binding to RANKL and thus preventing it from activating the osteoclasts. In the absence of RANKL-RANK interaction, there is an inhibition of the proliferation, differentiation in the precursor osteoclasts, and survival of the mature osteoclasts by initiation of apoptosis, which is a type of programmed cell death. Thus, there is a bone formation without the bone resorption. Although, due to the homeostatic and physiological balance. The rate of bone formation is also slow. But over time, its therapeutic benefits add up that leads to the reduced risk of osteoporosis. This is diagrammatically presented in Figure 2 .
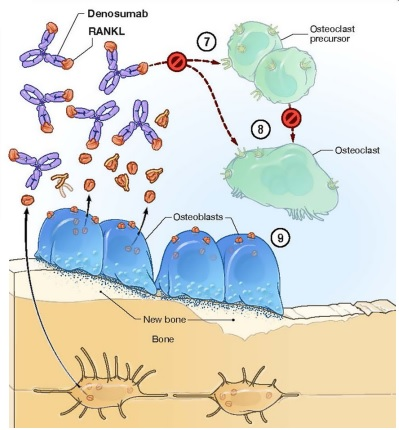
Figure 2 The Denosumab (as shown in purple) interacts and binds with the RANKL, which are produced and released by the osteoblasts. After binding, the interaction of the RANKL with RANK on the osteoclasts is prevented thus the resultant activation of osteoclasts is also prevented and as a result bone resorption is eliminated. These eliminated step, as a result of Denosumab treatment, are indicated by 7, 8 and 9 in this figure.
This inhibitory action of Denosumab is associated with the normal heathy bone structure at the tissue level, as evident from the histologic research studies. Moreover, it has also been studies that the five years prolonged Denosumab treatment is linked with the physiological level of mineralization of the bone matrix. Furthermore, when data from several independent research studies were plotted together. Overall, the most uniform therapeutic effect is caused by Denosumab when compared to Zoledronic acids and Alendronate. This is graphically illustrated in Figure 3 .
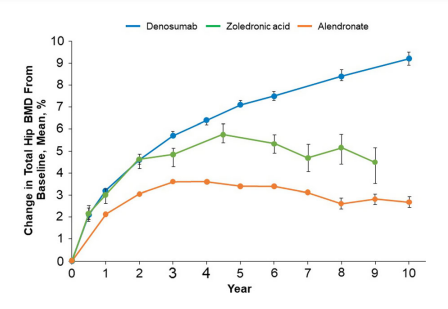
Figure 3 The uniform therapeutic effect, in terms of increased Bone Mineral Density (BMD), is shown by Denosumab treatment.
Interestingly enough, the effects of Denosumab treatments are reversible in nature. That essentially means that if Denosumab treatment is discontinued for any reason, same therapeutically positive effects, i.e., increased bone mineral density (BMD), can be effectively achieved by restarting the Denosumab treatment. This is scientifically proven fact, which is graphically illustrated in Figure 4. In this Figure 4, Denosumab treatment was the most effective in improving the bone mineral density.
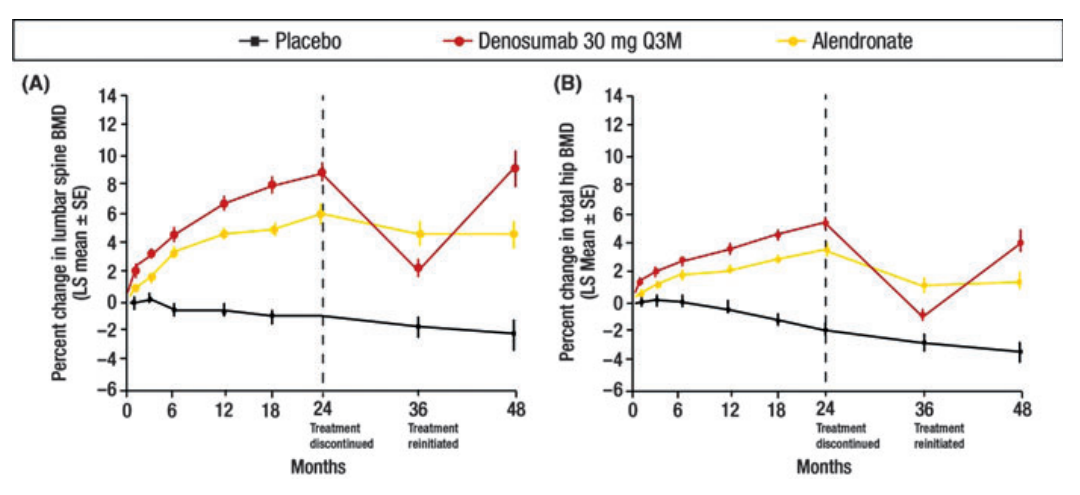
Figure 4 The effects of Denosumab and Alendronate treatments on the Bone Mineral Density (BMD) in the spine (vertebral bones) and hip (non-vertebral bone), as compared to the placebo control. Both Alendronate and Denosumab were the potential therapeutic agents in this scientific study. The Denosumab treatment was more reversible in nature than the Alendronate treatment, both in vertebral and nonvertebral bones, spine and hip.
In the phase 2 clinical trials, it has been shown that the amount of the c terminus collagen fibers decreased followed by the Denosumab treatment. This effect is reversed by the discontinuing Denosumab treatment. Then, this effect is again gained by retreating by Denosumab treatment. This is evident from Figure 5.
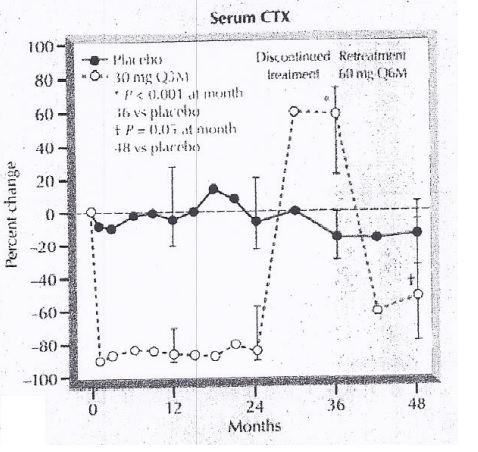
Figure 5 The correlation between the Denosumab treatment and the amount of c terminus Collagen fibers in the serum.
In the phase 2 clinical trials, it has been shown that the amount of the bone specific alkaline phosphatase decreased followed by the Denosumab treatment. This effect is reversed by the discontinuing Denosumab treatment. Then, this effect is again gained by retreating by Denosumab treatment. This is evident from Figure 6.
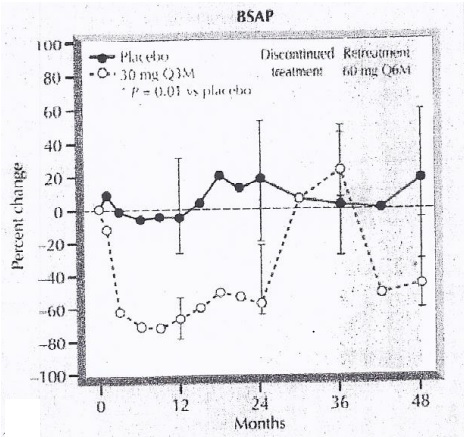
Figure 6 The correlation between the Denosumab treatment and the amount of Bone Specific Alkaline Phosphatase (BSAP).
From the results of the phase 2 clinical trials, the bone mineral density is found to be increased in the lumber spine as well as in hip bone as treated with Denosumab. It is evident from Figure 7.
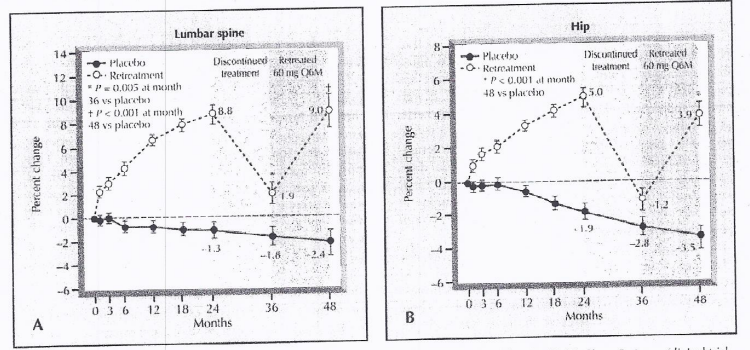
Figure 7 The Bone Mineral Density of the Lumber Spine and Hip bone, followed by the Denosumab Treatment.













コメント