LX1606
LX1032, LX1606, LX1606 Hippurate and are the various names used in scientific literature for the Telotristat Etiprate (TE). It is a small and new molecule. Its molecular structure is diagrammatically depicted in Figure 1.
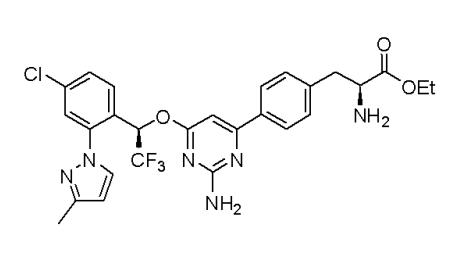
Figure 1 The Molecular Structure of Telotristat Etiprate (TE)
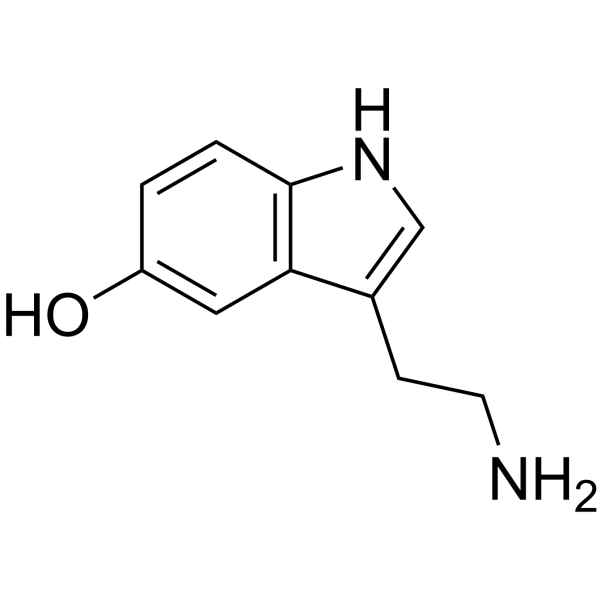
Telotristat Etiprate inhibits tryptophan hydroxylase (TPH), i.e., both of its optical isomers, TPH1 and TPH2 as indicated by a bold red cross in Figure 2, which is the enzyme that actively participates in the biosynthesis of 5-Hydroxytryptamine (5-HT), which is also known popularly as serotonin, as a rate-limiting enzyme in the body. This essentially means that the overall product of this serotonin biosynthesis, i.e., serotonin, is going to be determined as well as controlled by the activity of tryptophan hydroxylase enzyme. In simpler words, the amount of serotonin gets increased by activating tryptophan hydroxylase enzyme and the amount of serotonin gets decreased by inhibiting tryptophan hydroxylase enzyme. Plasma Serotonin is produced in high levels, in those who are suffering from carcinoid syndrome. Thus, by inhibiting tryptophan hydroxylase enzyme via Telotristat Etiprate treatment, the major but dependent signs of carcinoid syndrome, i.e., increased levels of serotonin and 5-hydroxyindole acetic acid in plasma and urine, respectively can be alleviated. 5-hydroxyindole acetic acid (5-HIAA) is chemically formed from serotonin from the catalyzing action of monoamine oxidase and aldehyde dehydrogenase in the periphery, for example, kidneys and liver, and is usually released in urine as a metabolite. In fact, using Telotristat Etiprate as a therapeutic agent for targeting the serotonin biosynthesis has been shown therapeutically positive results, across the scientific literature, in the animal models, i.e., specifically murine models, as well as in the clinical trials on the human subjects. In those scientific researches, Telotristat Etiprate treatment has been found to reduce the diarrhea which is associated with the carcinoid syndrome, which is due to the production of tumors by the Serotonin. in another clinical study, patient self-reported a relief, i.e., that was statistically adequate from bowel related symptoms after getting the Telotristat Etiprate treatment.
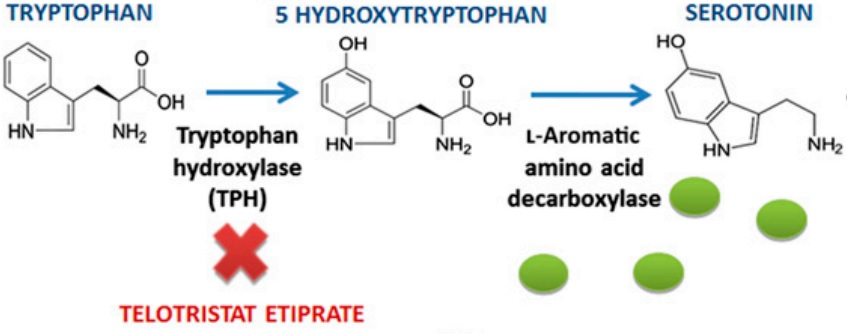
Figure 2 Telotristat Etiprate targets the rate determining or rate limiting step of the entire biosynthetic pathway for the Serotonin. Thereby, the synthesis of Serotonin can be effectively inhibited. The green circles are serotonin molecules
Let us look closely at the biosynthesis of Serotonin at the cellular level. The site of serotonin biosynthesis is the enterochromaffin cells and the neuroendocrine tumor cells/ carcinoid tumor. The biosynthesis of serotonin is a biochemical pathway, that essentially consists of two sequential biochemical reactions which are catalyzed by their own unique enzymes. In the first reaction, tryptophan acts as a substrate. It is an essential amino acid in the body, which is getting hydroxylated at the fifth carbon position via the catalytic activity of tryptophan hydroxylase (TPH) thus, forming 5-hyroxytryptophan. Both optical isomers of this enzyme TPH, i.e., TPH1 and TPH2, have the potential to catalyze this reaction. The only difference is that in brain cells, TPH2 catalyzes this reaction while in the peripheral tissues, TPH1 catalyzes this reaction. This is basically and essentially a hydroxylation reaction, i.e., addition of hydroxyl group to the substrate at any site. As stated earlier, this is the rate determining or rate limiting step of the entire biosynthetic pathway for Serotonin. Its significance in context to targeting it for the therapeutical application, is also elaborated in the above paragraph. In the second reaction, the same 5-hyroxytryptophan is getting converted into the final product serotonin, i.e., a monoamine, via the catalytic activity of L-Amino acid decarboxylase. This is basically and essentially a decarboxylation reaction, i.e., removal of carboxyl group from the substrate at any site. The final product, serotonin gets stored in the vacuoles of these cells, and gets released either into the blood or enteric tissues. All the molecular events and biochemical reactions, which are normally involved in the biosynthesis of serotonin are diagrammatically illustrated in Figure 3.
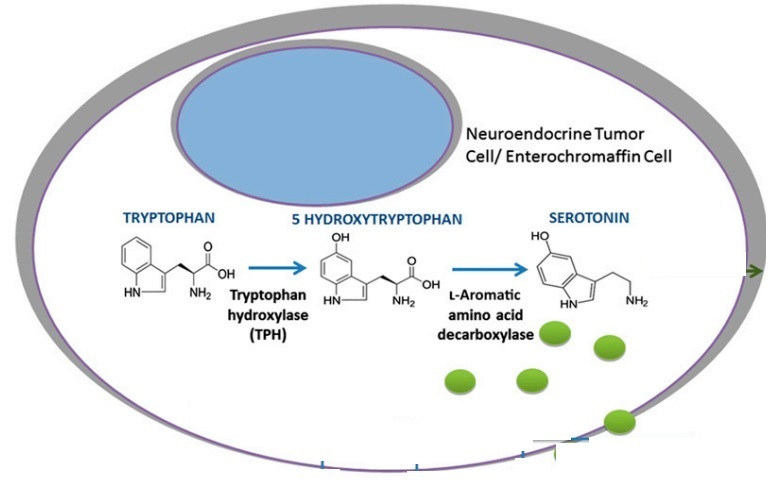
Figure 3 The Molecular Events involved in the Biosynthesis of Serotonin enterochromaffin cells and the neuroendocrine tumor cells. Two core biochemical reactions; hydroxylation of tryptophan (substrate) into a serotonin biosynthetic intermediate, which is followed by decarboxylation of that intermediate that leads to the formation of serotonin. The biosynthetic serotonin molecules are represented by the green balls
The biosynthetic serotonin molecule, then interacts with the different types of serotonin receptors, these are 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT-5, 5-HT-6 and 5-HT7. All of them are present in the human body in different sites, specifically cells. And, serotonin interaction with them leads to the activation as well as inhibition of several biochemical pathways. Thereby, serotonin is responsible for various biological functions in the body. These roles are diagrammatically illustrated in Figure 4, the most prominent roles are the increased gut mobility, vasoconstriction and fibrogenesis. Out of them, the former one is the result of serotonin release by the enterochromaffin cells. This chemical message is relayed through efferent and myenteric neuron, before getting translated into the increased gut motility which is manifested by the increased bowel movement. This is diagrammatically illustrated in Figure 5 . Apart from these, Serotonin also plays a key role in promoting the aggregation of platelets to the injury site and the wound healing.
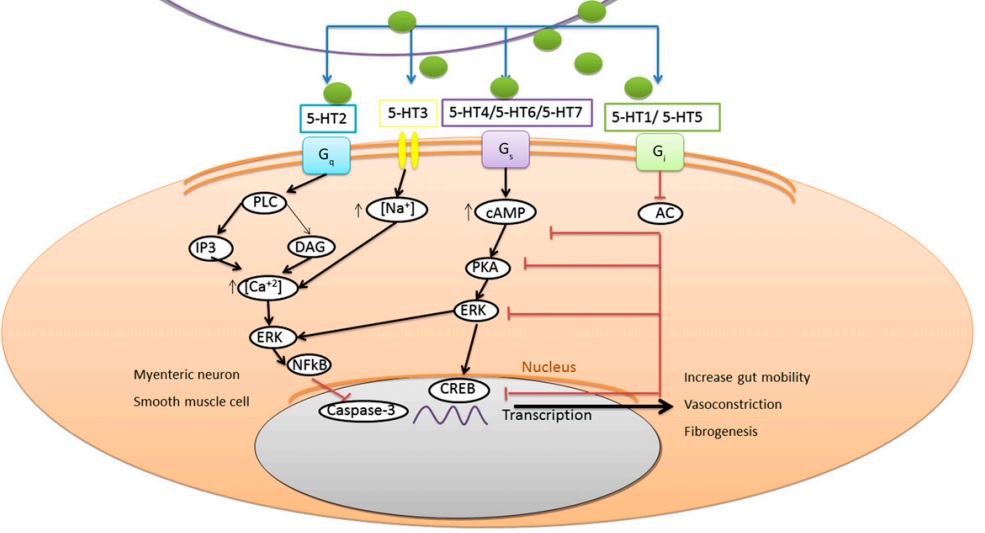
Figure 4 The biological functions, performed by Serotonin in the body
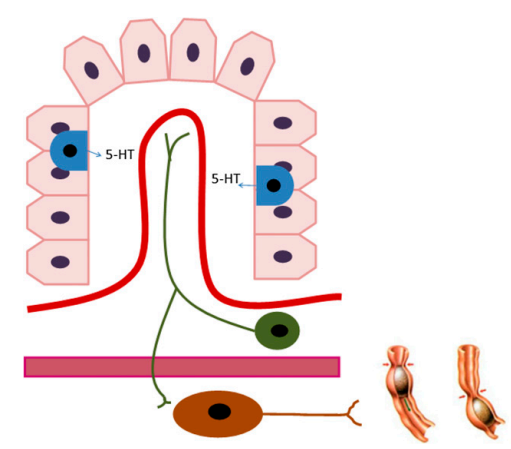
Figure 5 The increased gut motility, that leads to the increased bowel movement. enterochromaffin cells are indicated in blue, efferent neuron is indicated in green, while myenteric neuron is indicated in brown
Serotonin is naturally present throughout in the human body, i.e., central nervous system and peripheral tissues, but only peripheral Serotonin/ plasma Serotonin is effectively targeted and thus inhibited by Telotristat Etiprate, as Telotristat Etiprate cannot cross the blood-brain barrier. Approximately 90% of all the plasma serotonin synthesis is inhibited. This is important as Serotonin acts as a neurotransmitter of the central nervous system in normal physiology, and Telotristat Etiprate could be therapeutically used without any side effects on the vital normal functioning of the brain and spinal code. This is the main driving force that Telotristat etiprate is the first and only chemical compound to enter third phase of the clinical trials so far, as an inhibitor of the Serotonin Biosynthesis. Therefore, there are high chances that it could be used as an effective therapeutic treatment against those disease which are characterized by the upregulated synthesis of Serotonin, especially carcinoid syndrome.
So far, it is preliminary therapeutically orally administered as a produg in the clinical trials. This prodrug is the hippurate salt of Telotristat, which is ethyl ester in nature. That means, it primarily enters the body as an inactive drug with no pharmaceutical action but later on it is converted into its active form by the body’s normal metabolism. Specifically, for Telotristat Etiprate, it is converted into its pharmaceutically active form, which is Telotristat as a metabolite by the catalytic activity of the esterase enzymes, more specifically carboxylesterases, in the body.












コメント