Streptozocin
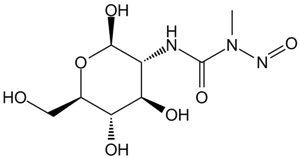
The soil-dwelling bacterium Streptomyces achromogenes is the source of streptozocin, also referred to as streptozotocin. It is structurally related to glucose and is a member of the nitrosourea chemical class. Because of its similarity to glucose, it is easier for pancreatic beta cells to absorb and causes a selective buildup in the pancreas. Streptozotocin, also known as streptozocin (INN, USP) (STZ), is an alkylating antineoplastic drug that occurs naturally and is especially harmful to the beta cells of mammals' pancreas that produce insulin. It is used in medicine to treat specific islets of Langerhans tumors. It is also utilized in medical research to create an animal model for numerous low dosages of type 1 or type 2 diabetes, hyperglycemia, and Alzheimer's disease. The 2D molecular structure of streptozocin is shown in Figure 1.
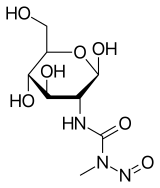
Figure 1: The molecular structure of Streptozocin
An animal model was initially used to demonstrate the diabetes generated by STZ. Based on the previous experimental model, it is usually given as a single intravenous dose of 40–60 mg/kg of body weight . Streptozotocin enters the pancreatic beta cells by glucose transporter 2 (GLUT-2) shown in figure 2. Steptozotocin works on the beta-cell by DNA alkylation triggered by the methyl nitrosourea moiety. DNA fragmentation is caused by the methyl group transfer of streptozotocin, which damages DNA. Poly ribosylation is triggered by DNA damage, resulting in decreased amounts of NAD and ATP and potentially leading to beta-cell death. With it, malignant insulinomas are treated. The low affinity glucose transporter GLUT-2 of beta-cells carries STZ into the cell, where it causes DNA alkylation and irreversible cell death. Four significant biological properties of streptozotocin are revealed by its biological actions, which include antibacterial, beta-cell (beta)-cytotoxic, oncolytic, and carcinogenic properties. Streptozotocin (2deoxy-2-[3-methyl-3-nitrosourea] 1-Dglucopyranose) can be separated into two anomeric forms by chromatographic means, and (HPLC) . The solubility of STZ is limited in water, ketones, and lower alcohols, but it is mostly soluble in polar organic solvents. This dissolves in water at a rate of 50 mg/mL, yielding a clear to slightly hazy solution that is pale yellow in color. In aqueous STZ solutions, there is a rapid mutarotation to an equilibrium mixture of alpha- and beta-anomers. The most stable STZ solution is at pH 4, and stability rapidly decreases at lower or higher pH values. Recently prepared solutions have a slight straw tint and are clear. Numerous research associations recommend that the streptozotocin solution be provided "immediately" and no later than 15 to 20 minutes after dissolving (in citrate or acetate buffer, pH 4.5)
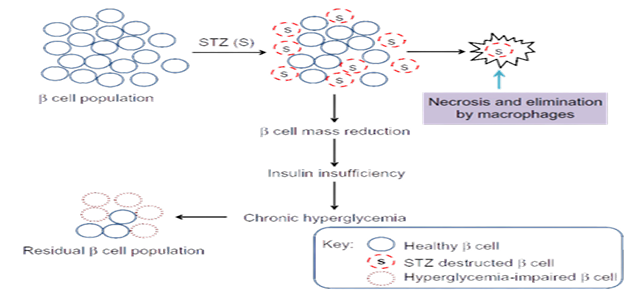
Figure 2: Mechanism of action of Streptozocin
Streptozotocin causes fast deoxyribonucleic acid (DNA) breakdown in Bacillus subtilis cells that are either resting or actively proliferating. Difference spectroscopy demonstrated that the antibiotic selectively interacts with mononucleotides that contain cytosine in vitro. The extremely small pH range of 5 to 5.5 is the only one where this interference happens is promptly reversed when the pH is raised or lowered. There was no discernible interaction seen between streptozotocin and isolated DNA. The potential is examined the relationship between cytosine residues in cellular DNA and streptozotocin even though they could occur infrequently, could serve as the main step that causes a DNA strand to break. Figure 3 illustrates how streptozotocin affects the synthesis of proteins and nucleic acids. It's clear that RNA and protein synthesis were unaffected in the 50 gg of streptozotocin per milliliter were present during the first 40 minutes following the antibiotic's administration. Upon during this time frame, only a slight inhibition of these procedures were visible. DNA synthesis is reflected in a partial but significant inhibition of 3H-thymidine incorporation. became visible 20 minutes after the medication was added. This shows that the interaction between streptozotocin and the creation or consistency of cellular DNA .
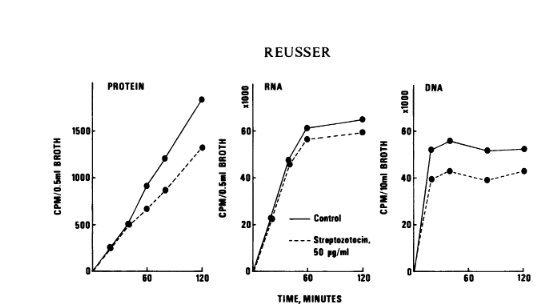
Figure 3: Effect of streptozotocin on protein and nucleic acid synthesis in B. subtilis.
Aspartic acid, uracil, and radioactive thymidine were used as pre-labels for these cellular fractions. The stability of the cellular nucleic acid and protein fractions was then assessed using the metric of leakage of integrated label from entire cells into the culture medium. Over the course of three to four hours of incubation, relatively minimal thymidine leakage was observed in the control culture (Fig. 4). The addition of the antibiotic caused massive thymidine leakage from the cells in the presence of streptozotocin to become visible right away. Throughout the whole trial, the rate of leakage remained impressively steady, increasing by almost six times over the control value. In both the streptozotocin-treated and control cultures, there was a noticeable amount of uracil label leaking into the culture medium . Nonetheless, the streptozotocin-treated culture's loss of uracil label was only slightly more than the control's for the first two hours. Over this time, the leakage rates were very stable. Uracil leakage increased in the antibiotic-treated culture after two hours. Regardless of the presence of streptozotocin, cells prelabeled with aspartic acid expelled only trace amounts of label into the culture medium. After about an hour, the streptozotocin-containing culture showed even somewhat less label degradation than the control .
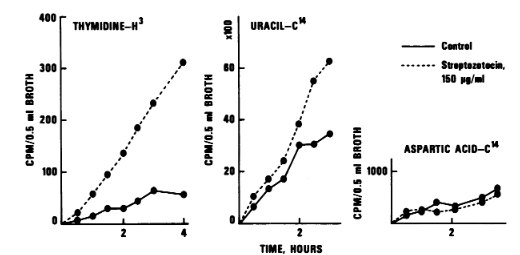
Figure 4: Effect of streptozotocin on stability of prelabeled nucleic acid or protein fractions in B. subtilis.
In the cell-dividing experiments, cells were first pre-labeled for two to three generations with 3H-thymidine and subsequently treated with streptozotocin in ordinary glucose-salts media for varying lengths of time. We extracted cellular DNA from protoplasts. To reduce DNA breakage during the isolation procedure, small amounts of protoplast suspension were lysed immediately on alkaline sucrose grains. Bacterial DNA was extensively broken down in the cells after 30 minutes of exposure to streptozotocin (Figure 5). The gradient profiles that were obtained were reduced to acid-soluble material and pieces of arbitrary length from cells treated with streptozotocin do not exhibit a distinct peak, indicating that the bacterial DNA. The control gradients demonstrate that the majority of the DNA from untreated cells settled in a small area toward the tube's bottom. After being exposed to the drug for 30 minutes, the cells were twice cleaned before being re-incubated for 90 minutes in new medium without the use of a tracer or antibiotic. A tiny peak was seen at the tube's bottom under these circumstances, and it represents material whose sedimentation velocity is the same as that of the control DNA (Figure 5). This implies that during reincubation following drug removal, either some de novo synthesis of DNA from tagged nucleotides or some rejoining of smaller DNA fragments by DNA repair enzymes had occurred.
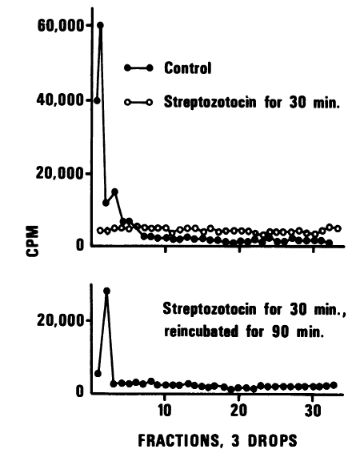
Figure 5: Effect of streptozotocin on sedimentation patterns of DNA from B. subtilis.
In B. subtilis cells, streptozotocin rapidly and extensively degrades cellular DNA. On the other hand, streptozotocin did not significantly degrade either synthesized polydeoxyribonucleotides or isolated DNA. Exonucleases may accelerate the DNA degradation process in cells once streptozotocin has caused a certain number of single- and double-strand breaks, despite the lack of an apparent rationale for this discrepancy. In B. subtilis cells, streptozotocin also preferentially suppresses DNA synthesis as opposed to RNA or protein synthesis. The partial suppression of DNA synthesis is likely due to a faster rate of DNA degradation caused by streptozotocin in comparison to de novo synthesis . Determining the correlation between the fast breakdown of the cellular DNA fraction and the described interaction of streptozotocin with cytosine-containing mononucleotides is a challenging task. It is possible that the pH in the cellular DNA portion could be low enough to allow the antibiotic and cytosine residues in DNA to interact, either at specific locations along the strands, throughout the strand, or during a certain physiological condition. In turn, this contact may cause strand breakage and result in DNA degradation.
One outstanding example of a naturally occurring substance with a variety of medical uses is streptozocin. Its dual function as a chemotherapeutic and antibiotic has made it an invaluable weapon in the fight against some cancers and in the advancement of our knowledge of diabetes. Its use in therapeutic settings may be improved and new applications may be found through ongoing research.














コメント