Trichostatin A
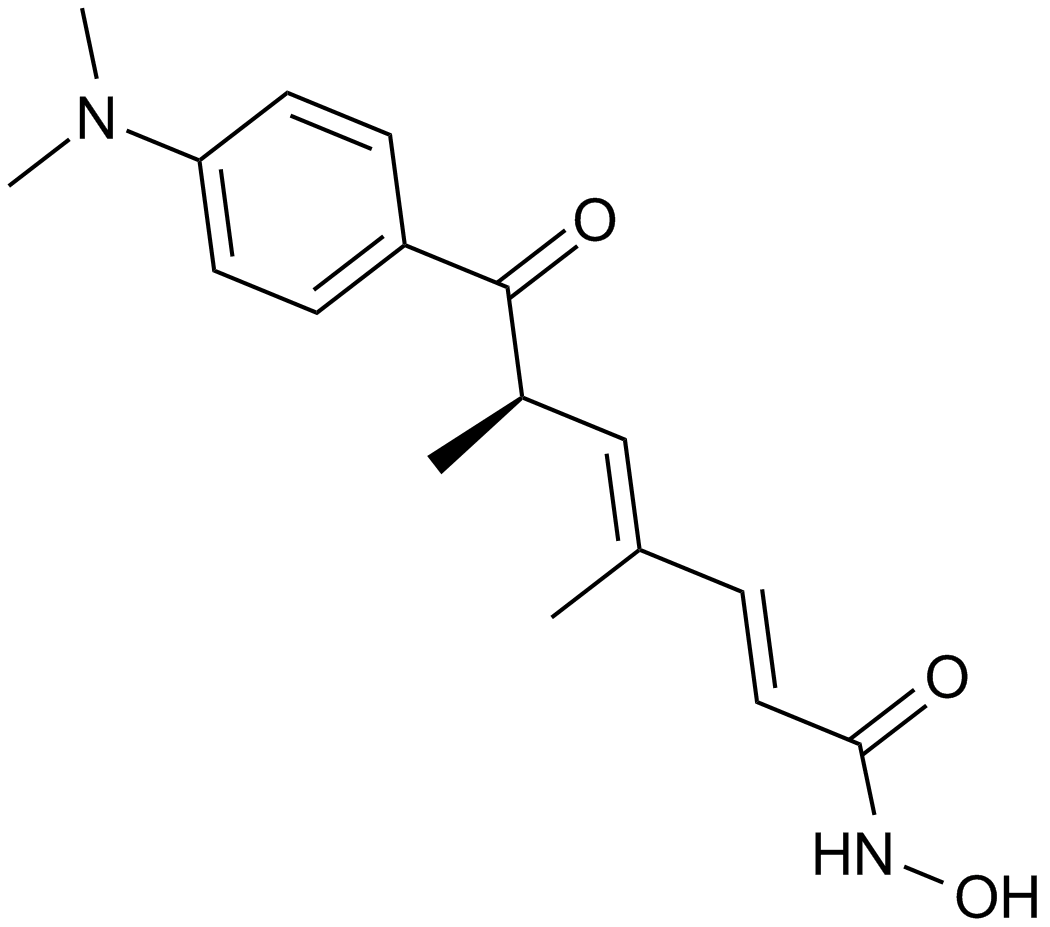
Trichostatin A (TSA) is a small molecule belonging to isohydroxamic acid. Its molecular structure showing its structural features is depicted in Figure 1.
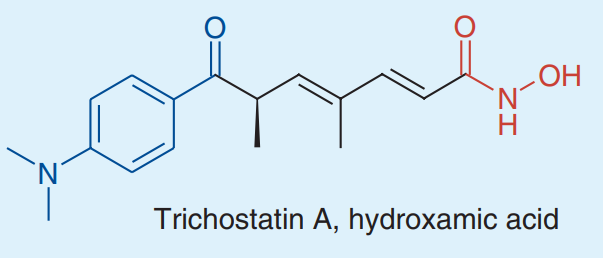
Figure 1 The 2D picture of the molecular structure of Trichostatin A compound, a hydroxamic acid. Blue part indicates Cap group which binds to the surface of enzyme (i.e., Histone Deacetylases (HDAC)), black part indicates linker group while red part indicates zinc binding group
Trichostatin A selectively interacts and effectively inhibits two classes of Histone Deacetylases (HDAC) namely Class Ⅰand Class Ⅱ, in a reversible manner; thus, it is classified as HDAC inhibitors (HDIs). On the molecular level, Trichostatin A binds to the zinc ionic bond present on the bottom of the tube-like structure of the Histone Deacetylase (HDAC) owing to presence of isohydroxamic acid and thereof Histone Deacetylases (HDAC) gets effectively inhibited. Being a Histone Deacetylases (HDAC) inhibitor, Trichostatin A has antitumor activity specifically breast cancer in a dose-dependent manner. Also due to the exact same reason, Trichostatin A treatment could also reduce the symptoms of limited cognitive abilities in schizophrenia.
Histone Deacetylases (HDAC) removes acetyl group from the lysine residues of the histones which acting in an antagonist relationship with the Histone acetyltransferases (HATs); leading to the compact packing of the histone molecules. In acetylation, the net positive electric charge on histone due to the lysine is neutralized. Histone molecules has a core consisting of four molecules (H2A, H2B, H3 and H4) and tail; specifically in the case of acetylated target protein, the DNA structure is relaxed and more genes could be transcribed. This molecular activity is a landmark of a number of cognitive and psychotic diseases specially schizophrenia. Only the amino acids of the histone tail carry out all the important post-translational modifications, e.g., methylation, ubiquitination, phosphorylation and acetylation in the newly expressed proteins. This is summarized in Figure 2.
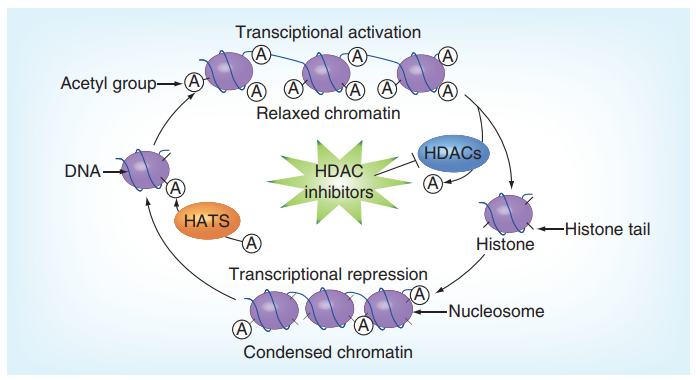
Figure 2 The Histone Deacetylases (HDAC) along with its antagonist enzymes Histone acetyltransferases (HATs) keep a balance in the transcriptional activity within the nucleus. In the presence of inhibitors of Histone Deacetylases (HDAC), DNA molecule is relaxed and more transcriptional activity is observed than the homeostatic level.
Trichostatin A also acts as anti-inflammatory drug against sepsis as it promotes Macrophage polarization (a specific phenotypic change leading to the formation of M2-polarized macrophages, also known as tumor associated macrophages (TAMs)) and autophagy as scientifically shown in the cecal ligation and puncture (CLP) mice model. Sepsis is a deadly disease characterized by a poor and dysregulated body’s immune response to the infectious agents. Macrophage polarization is highly dependent on particular stimuli present in the microenvironment; the resultant M2 phenotype macrophages are regulated by signal transducer and activator of transcription 3 (STAT3), peroxisome proliferator-activated receptor gamma (PPARγ) and interferon regulatory factor 4 (IRF4). The M2-polarized macrophages produce anti-inflammatory mediators, such as Interleukin 10 (IL-10) and Interleukin 4 (IL-4); thus, inflammation ends and body tissues begin to remodel. This finally leads to the treatment of sepsis which is also a risk factor for liver injury.
Trichostatin A also acts as neuroprotective. Hyperactive Histone Deacetylases (HDAC) causes neurodegeneration and alcohol consumption upregulates Histone Deacetylases (HDAC); while Trichostatin A could inhibit Histone Deacetylases (HDAC) thus it acts as neuroprotective even in alcohol use disorders (AUDs).
In a research study, Trichostatin A has been shown to reduce the adipogenesis. Excessive adipogenesis is a major landmark of the obesity (which is in itself a risk factor for a number of other diseases mainly cardiovascular disease, hyperlipidemia, diabetes, fatty liver and cancer, etc.) and defined as the proliferation and differentiation of the adipocytes that lead to the accumulation of adipose (fat tissue) at different sites inside the body. In the adipogenesis, the preadipocytes are proliferated and differentiated into adipocytes. This phenotypic change is mediated by a large number of transcription factors which upregulate the expression of a number of lipoproteins (also known as lipid proteins); the most important being peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer-binding protein α (C/EBPα) in this regard. PPARγ has its three subtypes PPARγ1 which expressed in relatively low level, PPARγ2 which is commonly expressed in the macrophages and the cells of the small intestine, and PPARγ3 which is abundantly present in the adipose tissue and essentially involved in the differentiation of adipocytes. As PPARγ is activated, it readily forms a dimer with retinoic acid receptor and then interacts with the peroxisome proliferator responsive element (PPRE) to regulate the transcription of the downstream target genes. The main target proteins of PPARγ are fatty acid-binding protein 4 (FABP4) and sterol regulatory element-binding protein 1c (SREBP1c). On the other hand, is C/EBPα whose three isomers including C/EBPα, C/EBPβ, and C/EBPδ, are of great significance when it comes to adipose differentiation specifically in the 3T3-L1 preadipocytes cell line. Overall, in the adipocyte differentiation, C/EBPβ, and C/EBPδ are upregulated; they both then upregulate PPARγ and C/EBPα and lead to the adipocyte differentiation.
The Trichostatin A treatment reduces the lipid accumulation which is the ultimate result of adipogenesis (shown by histologically stained micrographs in Figure 3 and graph bars in Figure 4). In addition, the Trichostatin A treatment promotes lipolysis, i.e., the biochemical breakdown of the lipid in the cell (as shown in Figure 5). The extent of lipolysis is estimated by quantifying the triglycerides and glycerol level in the cell; the triglycerides and glycerol are the final products of the lipolysis. Moreover, the genes like Leptin, FABP4, and SREBP1C are also downregulated by the Trichostatin treatment which were previously upregulated in the state of adipogenesis (as shown in Figure 6). Furthermore, the Trichostatin A treatment downregulates peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer-binding protein α (C/EBPα) (as shown in Figure 7); both are the promotors of lipogenesis and adipogenesis. Inhibition of adipogenesis via the Trichostatin A treatment is proposed to take place via AMPK pathway as Trichostatin A upregulates the AMPK expression (as shown in Figure 8). Overall, it also reduces the body weight, visceral adipose, volume of adipocyte, and weight of the visceral adipose tissue in mice model (as graphically shown in Figure 9).
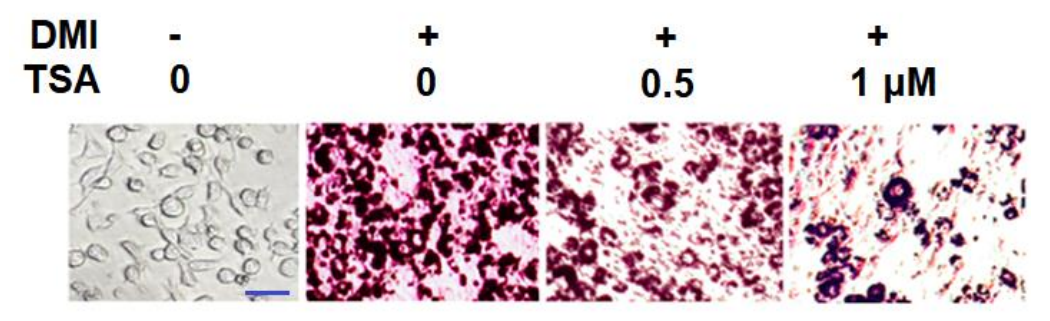
Figure 3 The Trichostatin A treatment reduces the lipid accumulation in a dose-dependent manner as indicated by the gradually decreasing Oil Red O staining
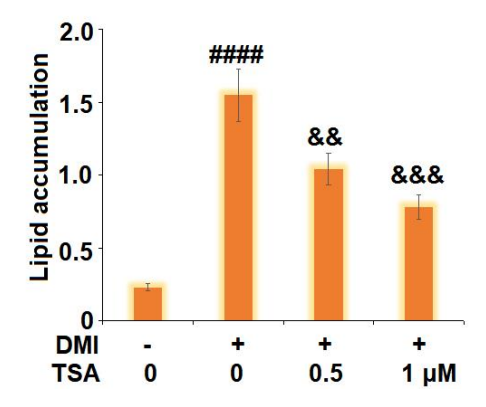
Figure 4 The Trichostatin A treatment reduces the lipid accumulation in a dose-dependent manner
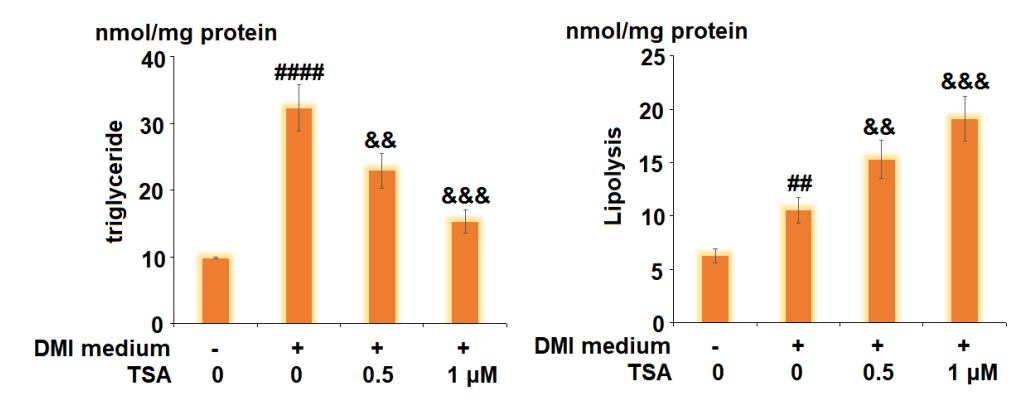
Figure 5 The level of triglycerides and glycerol is an index of lipolysis. The original triglycerides and glycerol amount in the cells is presented in left graph. After the Trichostatin A treatment, their amounts are increased and presented in right graph.
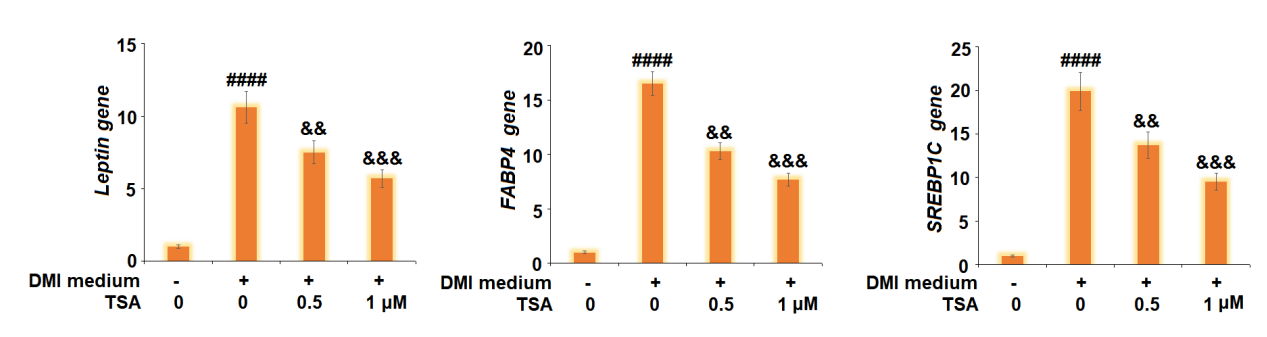
Figure 6 The Trichostatin treatment downregulate the expression of Leptin, FABP4, and SREBP1C genes.
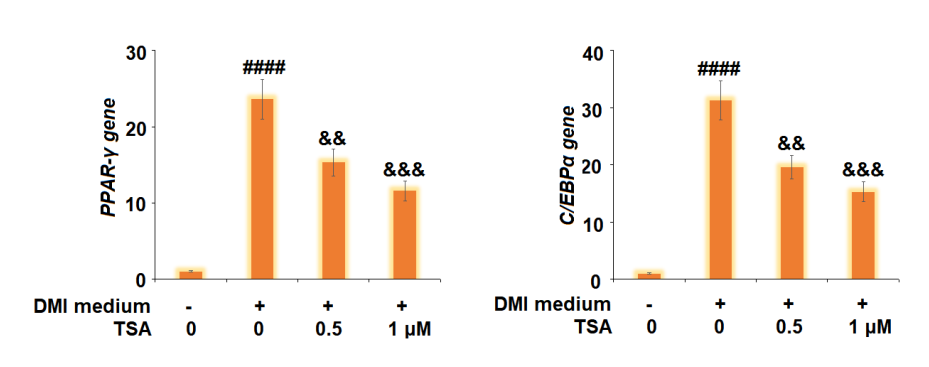
Figure 7 The Trichostatin treatment downregulates the transcription factors, i.e., peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT enhancer-binding protein α (C/EBPα), which promote the lipogenesis and adipogenesis.
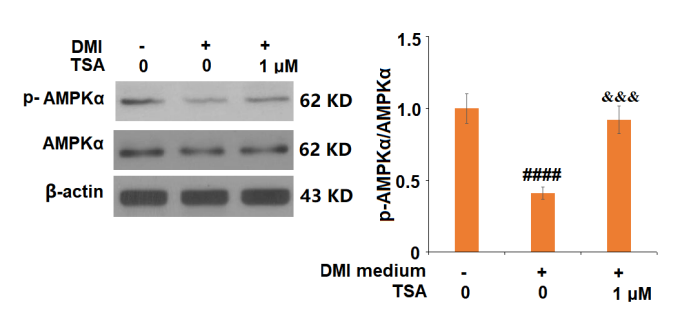
Figure 8 The Trichostatin A treatment upregulates the expression of AMPKα as well as of the p-AMPKα which is the phosphorylated form
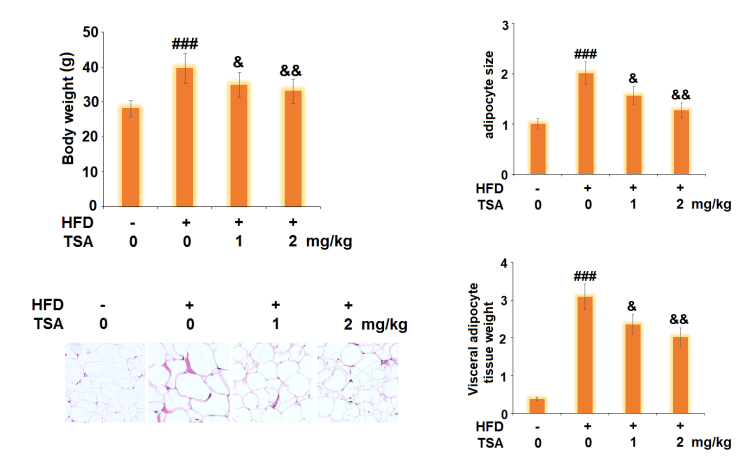
Figure 9 The Trichostatin A treatment reduces obesity in mice model via decreasing body weight, size of visceral adipose, size of adipocyte and weight of visceral adipose













コメント