Liproxstatin-1
The development of many diseases, such as injury from ischemia-reperfusion, cancer and neurological and cardiovascular disorders, is largely influenced by oxidative stress. When the antioxidant defences and the creation of reactive oxygen species (ROS) in the body are out of balance, oxidative stress develops. Recent attention has been drawn to ferroptosis, a type of controlled cell death that is specifically vulnerable to oxidative stress . Liproxstatin-1 (Lip-1) is a potential drug for the treatment of conditions linked to oxidative stress as it has demonstrated exceptional effectiveness in reducing ferroptotic cell death.
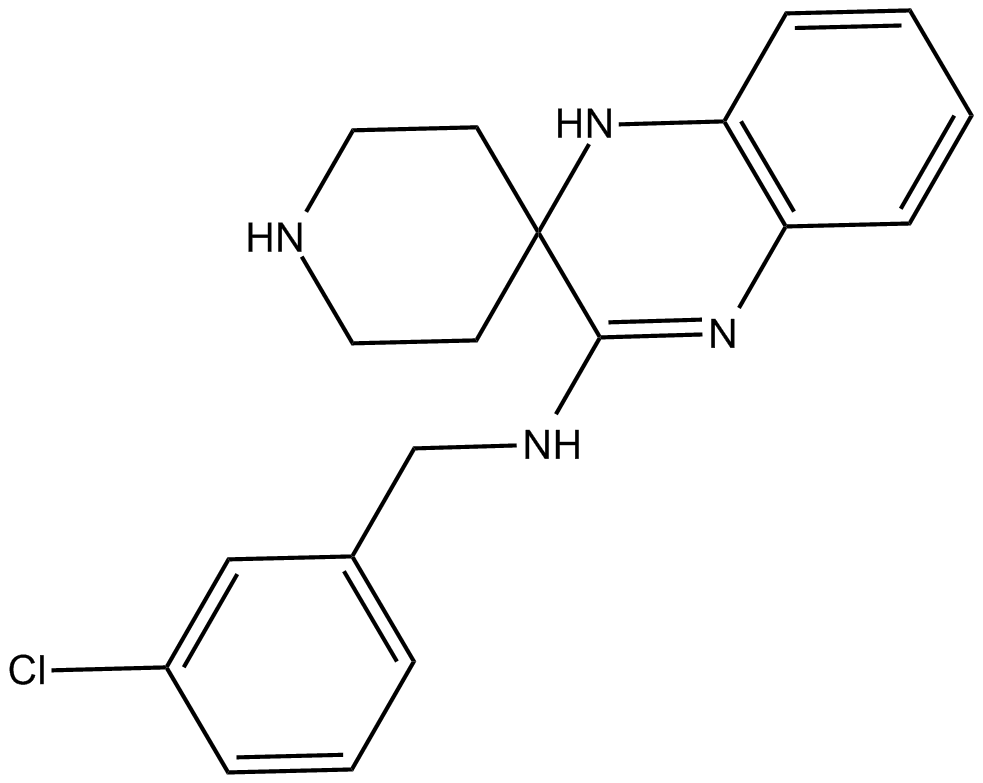
The chemical name for Liproxstatin-1 is N-[(3-chlorophenyl)methyl]-spiro[piperidine-4,2'(1'H)-quinoxalin]-3'-amine and has a chemical formula; C19H21ClN4 with molecular weight of 340.8 g/mol. Liproxstatin-1 is a powerful inhibitor of ferroptosis, a radical scavenger and cardioprotective agent, it has an IC50 value of 22 nM. The chemical structure of the Lip-1 is shown in the figure 1 below.
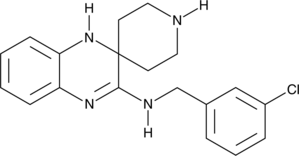
Figure 1: Chemical structure of the Liproxstatin-1;
A lipid-repair enzyme GPX4, glutathione peroxidase 4, controls the iron-dependent, regulated cell death known as ferroptosis, fueled by acute peroxidation of lipids. It depends on the overproduction of ROS (reactive oxygen species) and iron however is not dependent on necrosis, autophagy or caspase-mediated cell death. It has been demonstrated that a variety of ferroptosis regulators as well as inhibitors control ROS generation in an iron-dependent mechanism. The two classes identified as stimulants of ferroptosis are class 1 inducers; including sulfasalazine, erastin and sorafenib while class 2 inducers; include Ras-selective lethal 3 (RSL3) and FIN56. Numerous pathogenic processes, including ischemia-reperfusion (I/R) injury, acute kidney damage, neurological damage and cancer, have been linked to ferroptosis. In addition, a recent study claimed that ferroptosis is linked to the pathophysiology of injury from I/R and that glutaminolysis inhibitors could be used as a possible therapeutic target since they shield the heart from I/R injury.
The reliance on cystine absorption, glutathione production and GPX4's role in preventing lipid ROS buildup are characteristics of the overall ferroptosis process as per Cao and Dixon . When these mechanisms are interfered with, either by preventing cystine uptake or by suppressing glutamate-cysteine ligase (GCL), a synthetic enzyme, it results in cell death that is characterised by the buildup of fatal lipid peroxides (Figure 2).
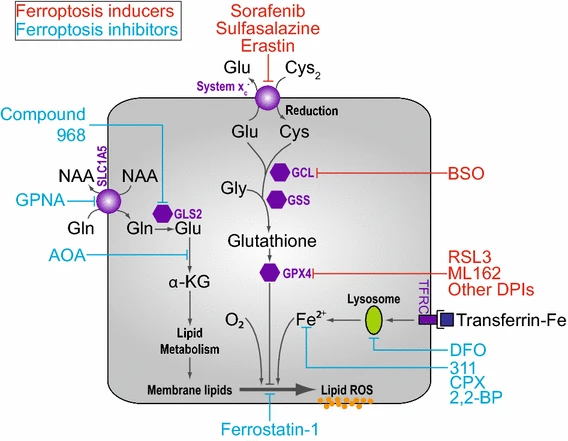
Figure 2: An overview of the working pathway of ferroptosis;
Since they may quickly and selectively regulate biological reactions, small organic molecules can be a significant addition to genetic tools which lead to the discovery of new types of controlled cell death. These compounds which can either trigger or prevent ferroptosis present potential therapeutic pathways. According to a study by Dong et al. (2015), cell death brought on by Erastin, a RAS-selective oncogenic molecule, appeared to be distinct compared to apoptosis, necrosis and autophagy in terms of morphology, biochemistry and genetics. this type of cell death was then maned as ferroptosis (Figure 3).
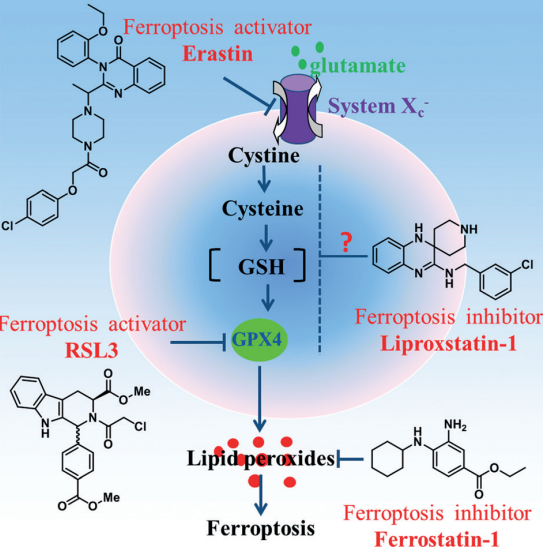
Figure 3: Small molecular probes suppress ferroptosis;
Two substances, Ferrostatin-1 and Liproxstatin-1, have been discovered to be effective inhibitors of ferroptosis. They suppress ferroptosis which is stimulated by tiny substances like Erastin and RSL3. The capacity of these compounds to block lipoxygenase enzymes was first assumed to be the cause of their efficiency; however, showed that their main mode of action is as radical-trapping antioxidants (RTAs), which is how they work best. Additionally, it was demonstrated that Fer-1 as well as Lip-1 interact with peroxyl radicals more efficiently in phosphatidylcholine lipid bilayers while being slower than α-tocopherol (a kind of vitamin E). This is consistent with their greater effectiveness as ferroptosis inhibitors than α-tocopherol.
Liproxstatin-1 localises to cell membranes, where it interacts with lipid molecules preventing their peroxidation. It also interacts with other biological components to exert its effects. Since mitochondria are a major source of ROS, these important organelles are shielded from oxidative damage by mitochondria's protective properties. By maintaining the availability of reduced glutathione, Lip-1 indirectly supports the function of GPX4, a selenoprotein that is essential for neutralising lipid peroxides. Moreover, Liproxstatin-1 decreases the amount of free iron ions by chelating iron, preventing them from taking part in the Fenton reaction that produces highly reactive hydroxyl radicals. It was discovered, while evaluating a library of small molecules, that Lip-1 can decrease ferroptosis in cells, for instance in the preclinical models of ischaemia-reperfusion (I/R) induced hepatic injury and GPx4-l- mice. However, future studies are suggested to work on disclosing the exact working mechanism of Lip-1.
Liproxstatin-1 is a derivative of arylalkylamine, in contrast to traditional flavonoids and associated natural compounds, that utilise a -OH group to trap the free radical, Liproxstatin-1 functions as a reducing agent to intercept and neutralise lipid peroxide via passing hydrogen from the -NH group to the free radical. Additionally, the absence of benzene makes the substituted group essential for its biological function. The study used density functional theory (DFT) computations and molecular dynamics (MD) simulations to examine the process of H-transfer from -NH group to a condensed form of a lipid peroxide radical.
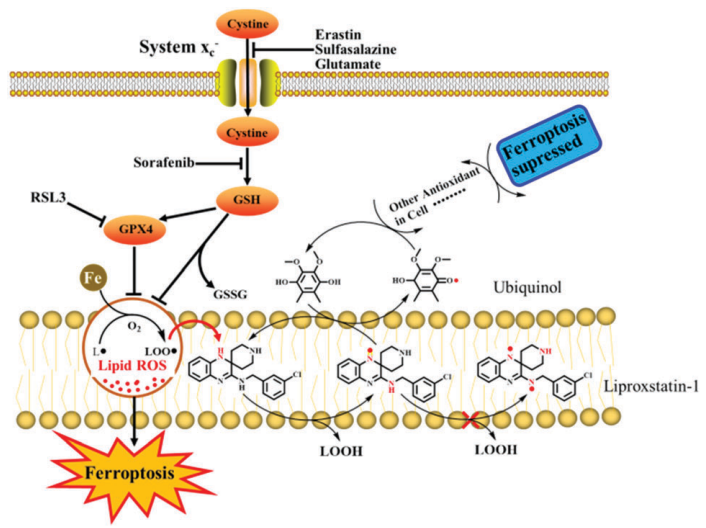
Figure 4: The potential scheme via which Liproxstatin-1 may suppress ferroptosis;
Based on the energy barrier for hydrogen atom transfer, the study discovered that the -NH groups in Lip-1 can be ordered as 1'-NH > 2'-NH 3'-NH. The Lip-1 has a high free radical scavenging activity because of its low potential barrier energy (that is 13.45 kcal/mol) for the transfer of hydrogen atoms.
In the context of ischemia myocardium, a situation in which the heart muscle encounters decreased blood circulation and eventual reperfusion, the function and mechanism of Lip-1 is studied regarding its decreasing cell death activity. It was looked into how Lip-1, which prevents ferroptosis and encourages cell survival, works to preserve the heart. Lip-1 treatment at the beginning of reperfusion had multiple positive benefits on the heart, the researchers found, in an experimental model comprising isolated perfused mouse hearts subjected to I/R injury. The research findings suggest that Lip-1 therapy had an inhibitory effect against cardiac tissue damage as seen by a reduction in the extent of myocardial infarcts. Also, it has been shown to help keep the mitochondria's structure intact and an appropriate operation, which is crucial for the cardiac cells' ability to produce energy.
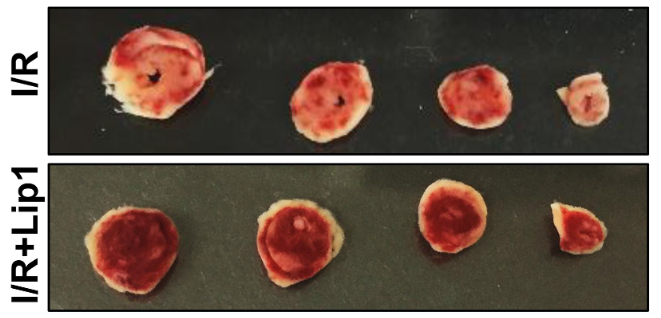
Figure 5: The cardiac slice image demonstrating reduced myocardial infarct size following post-ischemic Lip-1 treatment;
With the help of electron microscopy was displayed the ultrastructural examination of heart tissue demonstrated enhanced mitochondrial morphology along with contraction apparatus, which were isolated from ischemic and sham hearts, after post-ischemic Lip-1 administration.
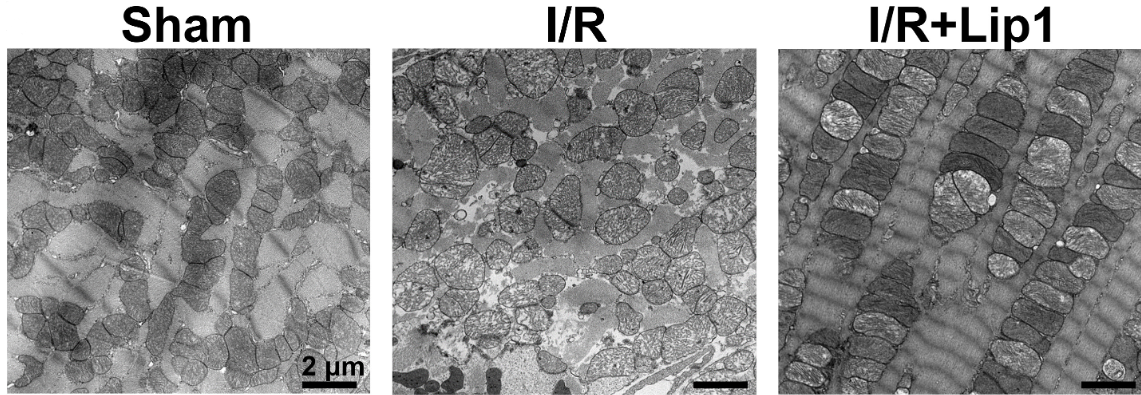
Figure 6: Image of the ultrastructural analysis via electron microscopy of heart tissue.
Thus, by preventing ferroptosis, lipostatin-1 (Lip-1) treats conditions associated with oxidative stress as it functions as a radical-sequestering antioxidant, preventing peroxidation of cell membranes along with mitochondria and lowering free iron ions. In numerous scenarios, including ischemia-reperfusion injury, it has demonstrated efficiency in preventing cell death.













Comments