RSL3
|
| Catalog No.GC12431 |
Glutathione peroxidase 4 inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
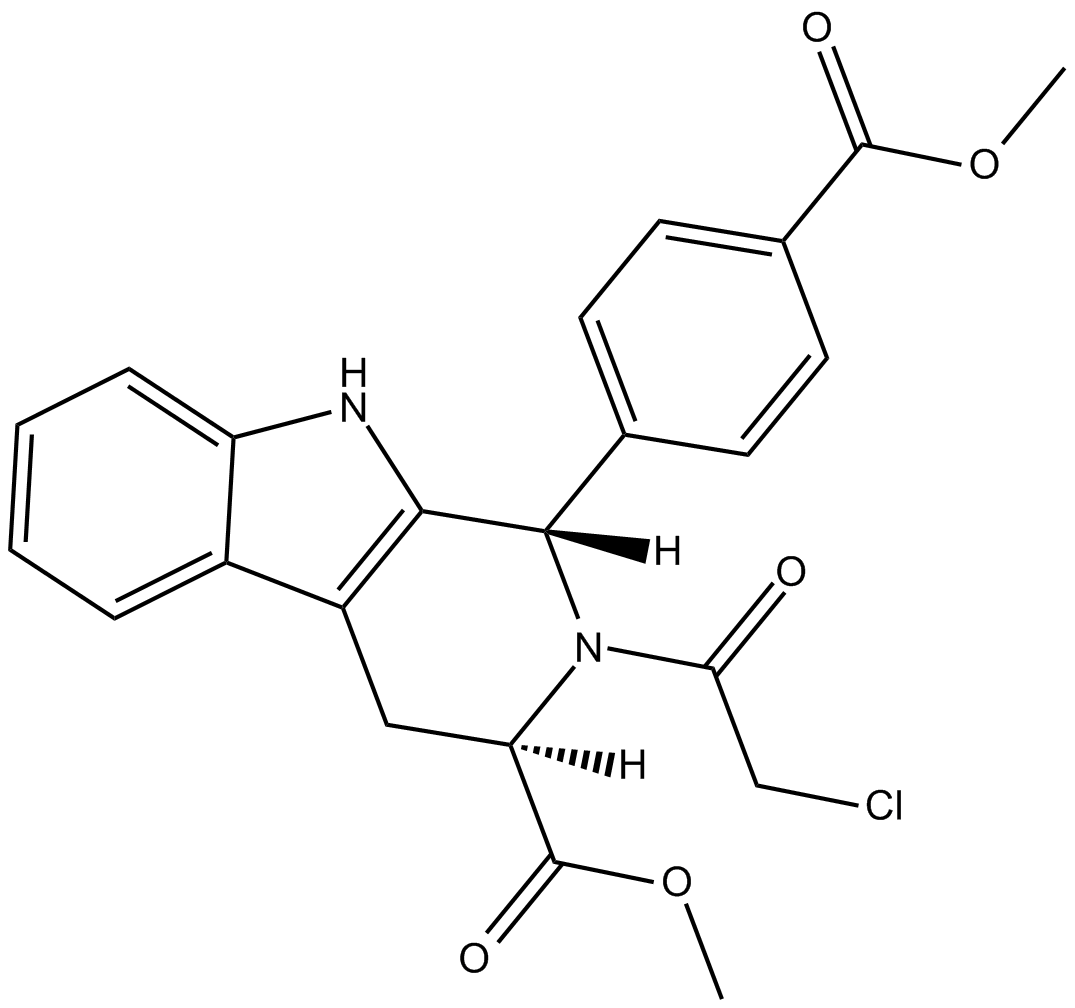
Cas No.: 1219810-16-8
Sample solution is provided at 25 µL, 10mM.
RSL3 is identified as a potent ferroptosis-triggering agent, which is dependent on the activity of GPX4. RSL3 behaved as an inhibitor of GPX4, reducing the expression of GPX4 and inducing ferroptotic death of head and neck cancer cell[1]
In vitro study demonstrated that RSL3 have a degree of synthetic lethality with oncogenic RAS. RSL3 showed rapid and potent ability to induce synthetic lethality with oncogenic RAS. RSL3 inhibited the growth of BJ-TERT/LT/ST/RASV12 and DRD cells as low as 10 ng/ml and started to kill sensitive cells as early as 8 h after treatment. The growth inhibitory effect and the selectivity of RSL3 were confirmed by trypan blue exclusion assay, which demonstrated the potency and selectivity of RSL3. Moreover, longer treatment with RSL3 had little effect on the viability of cells lacking oncogenic RAS, confirming the qualitative nature of RSL3’s selectivity.[2]
In vivo study determined that RSL3-induced ferroptosis could be enhanced by cetuximab with the mechanism of suppressing the Nrf2/HO-1 axis. A DLD-1 xenograft nude mouse model was established to further explored whether cetuximab promotes RSL3-induced ferroptosis in vivo. All the mice survived well after cell implantation or were treated with vehicle, RSL3, cetuximab, or RSL3 in combination with cetuximab. However, administration of RSL3 alone and treatment with both RSL3 and cetuximab led to a decrease in tumour size and tumour volume. Meanwhile, the relative levels of Nrf2 and HO-1 were decreased and that keap1 expressed was relatively increased after co-treatment with RSL3 and cetuximab.[3]
References:
[1]. Sui X, et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol. 2018 Nov 22;9:1371.
[2]. Shin D, et al. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018 Dec;129:454-462.
[3]. Yang J, et al. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021 Nov 13;12(11):1079.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *





