(S)-Ceralasertib (Synonyms: (S)-AZD6738) |
| Catalog No.GC34999 |
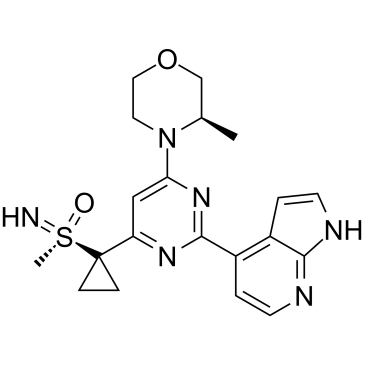
(S)-Ceralasertib((S)-AZD6738)은 특허 WO2011154737A1, 화합물 II에서 추출되며 2.578 nM의 IC50을 나타냅니다.(S)-Ceralasertib은 우수한 전임상 및 PK를 가진 강력하고 선택적인 설폭시민 모르폴리노피리미딘 ATR 억제제입니다. ) 특성.(S)-Ceralasertib은 수용성을 개선하고 CYP3A4 시간 의존적 억제를 제거하기 위해 개발되었습니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1352226-87-9
Sample solution is provided at 25 µL, 10mM.
(S)-Ceralasertib is extracted from patent WO2011154737A1, Compound II, exhibits an IC50 of 2.578 nM[1].(S)-Ceralasertib is a potent and selective sulfoximine morpholinopyrimidine ATR inhibitor with excellent preclinical physicochemical and pharmacokinetic (PK) characteristics.(S)-Ceralasertib is developed improving aqueous solubility and eliminates CYP3A4 time-dependent inhibition[2].
[1]. By Foote, et al. Morpholinopyrimidines as ATR kinase inhibitors and their preparation, pharmaceutical compositions and use in the treatment of cancer. PCT Int. Appl. (2011), WO 2011154737 A1 20111215. [2]. Foote KM, et al. Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutatedand Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J Med Chem. 2018 Nov 21;61(22):9889-9907.
Average Rating: 5 (Based on Reviews and 26 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















