Apatinib Mesylate
A small chemical molecule, Apatinib Mesylate, also named YN968D1, is an orally administered antiangiogenic drug developed by Shanghai Hengrui Pharmaceutical, China. YN968D1 specifically inhibits vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase. It blocks the pathways which lead to the downstream signalling of VEGFR-2 and reduces the density of cancer microvessels. When used against many experimental tumour models, Apatinib exhibits anticancer effectiveness. It prevents tumour angiogenesis by preventing the spread and development of vascular endothelial cells, it also inhibits other tyrosine kinases like c-Kit and c-Src. In numerous solid cancers, such as lung tumours, osteosarcoma, breast cancer and hepatocellular carcinoma, it has shown encouraging anticancer activity and manageable toxicities. Apatinib's potential therapeutic action, together with its safety profile and minimal toxicity, were all proven in the phase I clinical investigation.
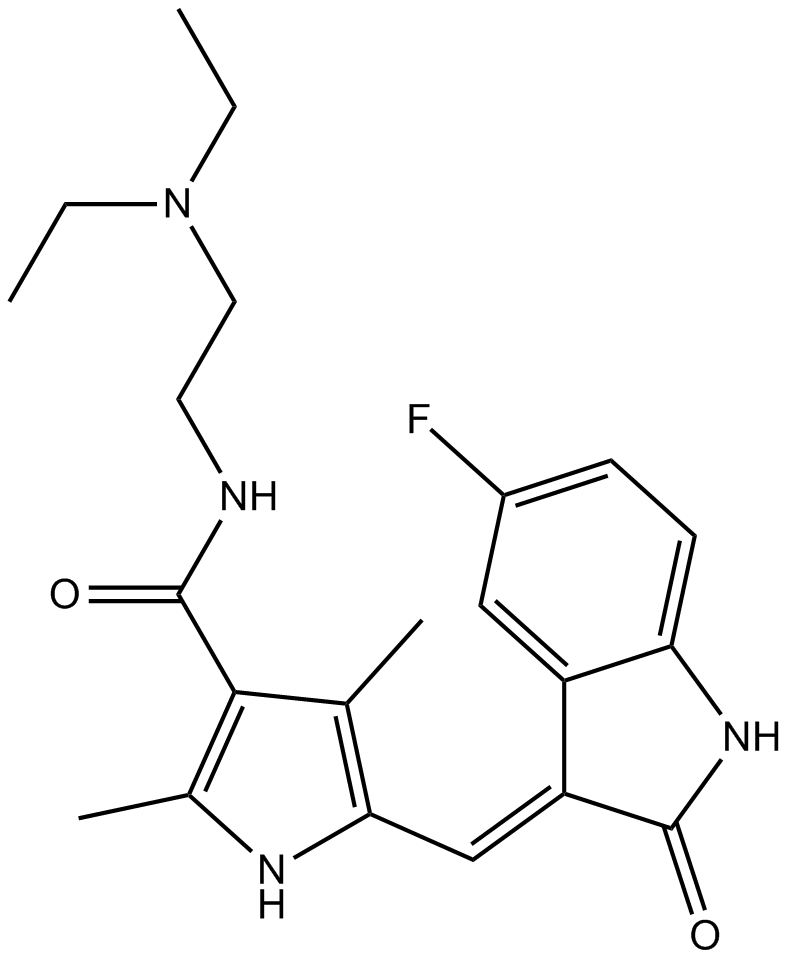
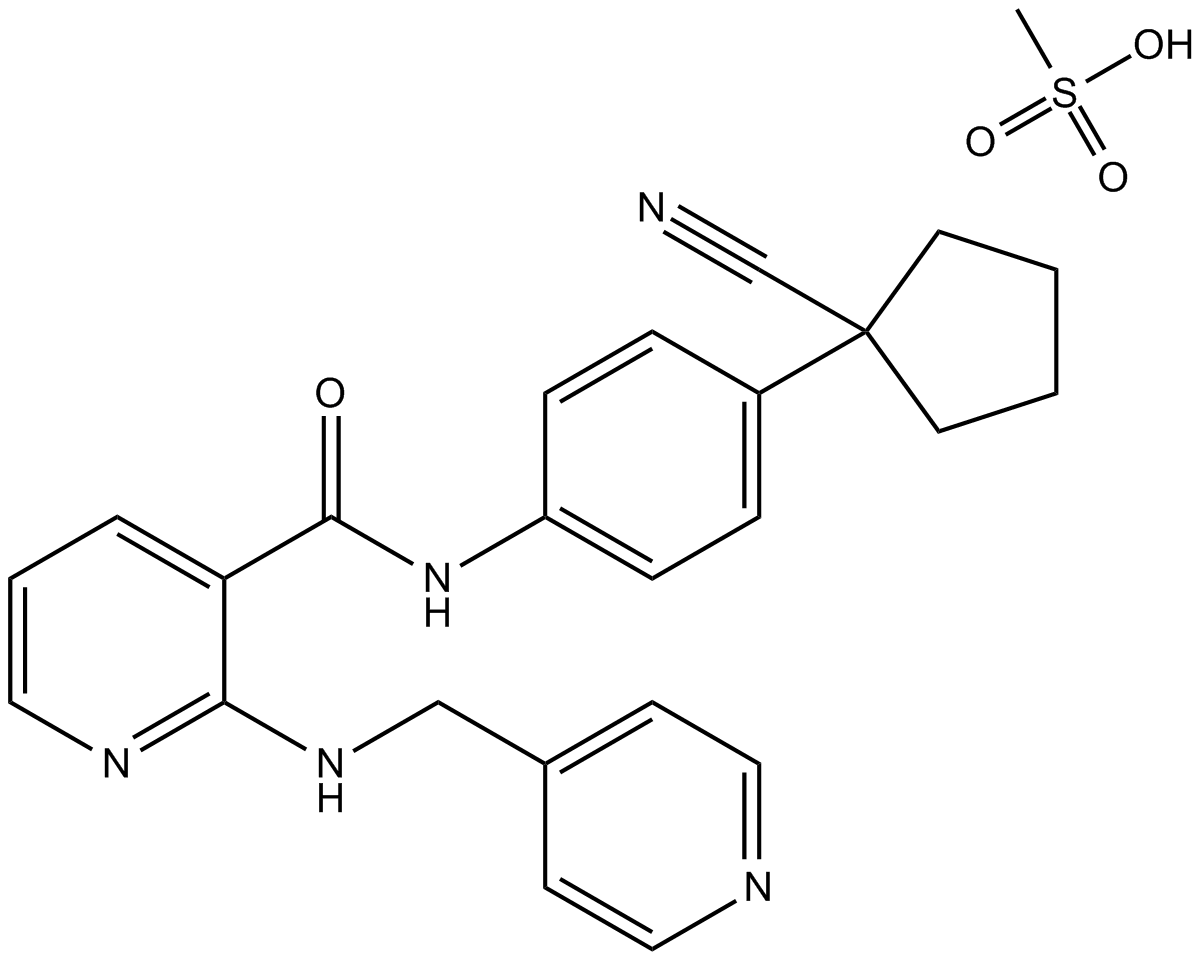
The chemical name for Apatinib is N-[4-(1-cyano-cyclopentyl) phenyl]-2-(4-pyridylmethyl) amino-3-pyridine carboxamide mesylate. It has a molecular formula C25H27N5O4S with a molecular weight of 493.58 Da (Zhang, 2015). The chemical structure of the drug I shown below (Figure 1).
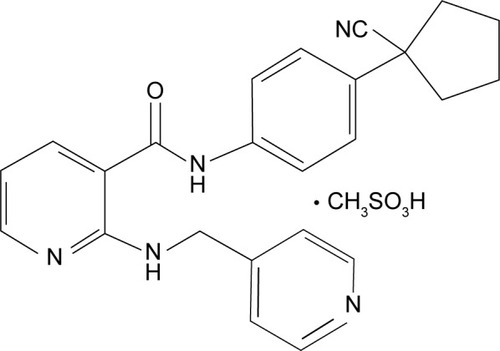
Figure 1: Chemical Structure of the Apatinib.
The pharmacokinetic analysis showed that the average half-life of Apatinib is 9 hours and the time of highest level of concentration in plasma is 4 hours, after dosage. In China, the suggested treatment dose of Apatinib, for individuals suffering from severe gastric cancer, is 850 mg/day.
An essential component for the onset and progression of tumors is angiogenesis which is linked to VEGF (vascular endothelial growth factor) and associated receptor VEGFR. The receptor family (VEGFR) has three subtypes (VEGFR-1, VEGFR-2, VEGFR-3) whereas the interaction of VEGFR-2 with the subtype of growth factor, VEGF-A, is said to be responsible for the development of blood vessels within solid cancers. After binding, VEGF-A dimerises the receptor and triggers the autophosphorylation of its tyrosine kinase. The drug, Apatinib mesylate, attaches to the cellular ATP binding pocket of the receptor with high specificity and inhibits phosphorylation of VEGFR-2. It prevents the vascular endothelium's following effects like cell growth, migration and survival. Thus, Aptatinib acts as an antiangiogenic agent by inhibiting this process as it has a 10 times stronger binding affinity, its IC50 is 2, for the receptor VRGFR2 compared to other anti-angiogenic agents like Sorafenib (IC50 = 90) (Figure 1).
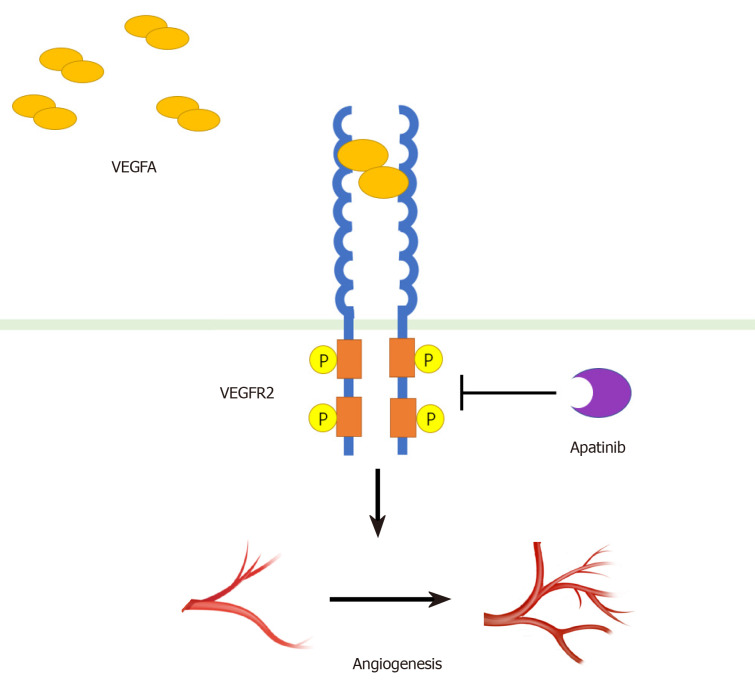
Figure 2: The schematic presentation of the mechanism of inhibition of VEGFR-2 by Apatinib
Patients with mutant EGFR lung adenocarcinomas (LA) showed resistance when treated with EGFR-tyrosine kinase inhibitors (EGFR-TKIs), due to only moderate efficacy of other drugs. The effectiveness of Apatinib Mesylate (also called rivoceranib) in treating patients who were resistant to EGFR-TKIs was assessed in light of the strong correlation among EGFR and VEGFR pathways as well as potential angiogenic activity. According to Fang et al. (2019), apatinib can be used to treat lung adenocarcinoma as it demonstrates suppression of several EGFR-TKI promoters, namely SRC, PDGFR, HMGB1, BEGF and others, which can explanations as how Apatinib treats EGFR-TKI-resistant LA.
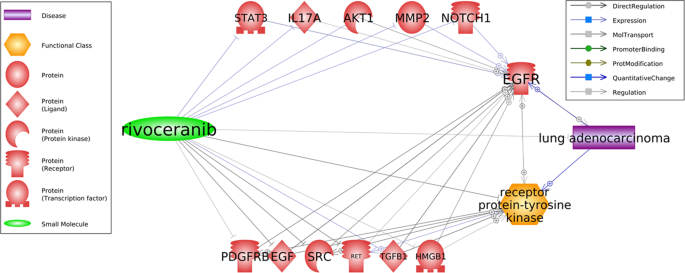
Figure 3: Potential pathways for the treatment of EGFR-TKI resistant lung cancer by Apatinib (rivoceranib).
Chi et al. (2022) studied the antitumor activity of Apatinib in combination with other cytotoxic medicines using oesophageal squamous cell carcinoma (ESCC) cells. The apatinib used to treat the ESCC cells in varied dosages showed that the drug inhibited cell proliferation in these cells by triggering cell death which was stimulated by the downregulation of Bcl2 expression. Additionally, the drug prevented the tumour cells' invasion and migration by drastically reducing the expression of the mesenchymal marker vimentin and the metastatic marker MMP9 (matrix metalloproteinase 9). Apatinib slowed the growth of malignant neoplasms by increasing the expression of the epithelial marker E-cadherin as per Huang et al. (2018). Additionally, cancer cell functions are impacted by the activation of the Akt/mTOR pathway. The drug decreased the proliferation of ESCC cells by reducing the expression of p-Akt and p-S6 and boosting the inhibitory effects on Akt and mTOR pathway.
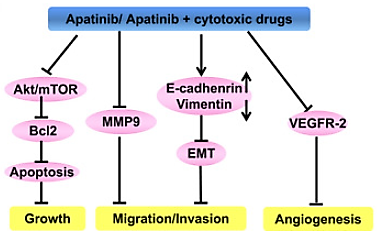
Figure 4: An illustration of how Apatinib works.
Apatinib was introduced to ESCC cell lines, KYSE450 and EC1, to investigate its effect on cell apoptosis and the outcome was determined through flow cytometry. The findings demonstrated that, in comparison to the control group, the drug enhanced apoptosis in cells of KYSE450 and EC1 cell lines (Figure 4). To further validate these findings, the alterations in apoptotic proteins of Apatinib-treated cells and the control group (DMSO treated) were examined and it was observed that Apatinib dramatically reduced Bcl2 protein expression in treated cells that were exhibited through Western blotting shown in Figure 5.
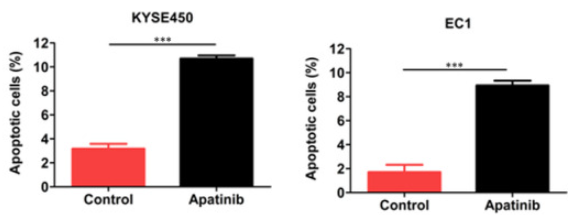
Figure 5: Cells showed apoptosis on treatment with drug.
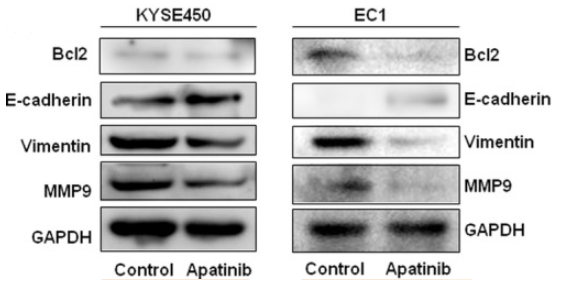
Figure 6: Expression of proteins (MM9, vimentin, E‐cadherin and Bcl2) in Apatinib-treated KYSE450 and EC1cells shown via western blot.
It was observed that human umbilical vein endothelial cells (HUVEC) are negatively affected by YN968D1 in terms of growth and migration within rat aortic ring, in vitro. In HUVEC, Apatinib reduced VEGF-stimulated phosphorylation of VEGFR-2/KDR in a dose-dependent manner. At the concentration of 0.1 μM, comparable to sunitinib (protein kinase inhibitor drug), YN968D1 entirely prevented VEGFR-2 activation. Concurrently was inhibiting the phosphorylation of ERK1/2, VEGF signalling (Figure 7).
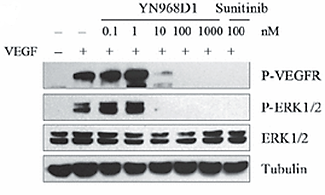
Figure 7: YN968D1 inhibited the phosphorylation of VEGFR and ERK1/2
Additionally, in Mo7e and NIH-3T3 cells, YN968D1, also in dose dose-dependent manner, reduced the phosphorylation of PDGFRβ and c-kit. However, YN968D1 needed somewhat greater concentrations than sunitinib to entirely inhibit both c-kit and PDGFRβ activations (Figure 8 A and B).
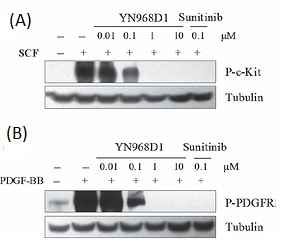
Figure 8: YN968D1 inhibited the phosphorylation of c-kit and PDGFR.
However, at 10 μM concentration, the drug did not affect EGFR and Her-2 phosphorylation as shown in Figure 9 (A and B).
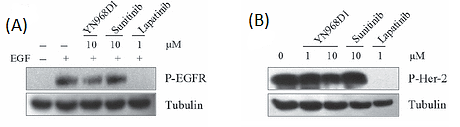
Figure 9: YN968D1 did not affect the phosphorylation of EGFR and Her-2.
In a study on acute lymphoblastic leukaemia (ALL), apatinib dramatically suppressed proliferation and promoted apoptosis in cells, in a time and dose-dependent manner, for both B and T lineage. In vitro, apatinib exhibits cytotoxic properties targeting primary cells in adult ALL whilst sparing healthy cells. Moreover, in a xenograft model, it inhibited ALL growth and survival, in vivo. The western blotting was used to examine phosphorylated-VEGFR2 (p-VEGFR2) in Jurkat and Nalm6 cell lines for ALL. Besides downregulating two downstream signals of VEGFR2, p-ERK and p-AKT, it showed that Apatinib significantly reduced VEGFR2 phosphorylation.
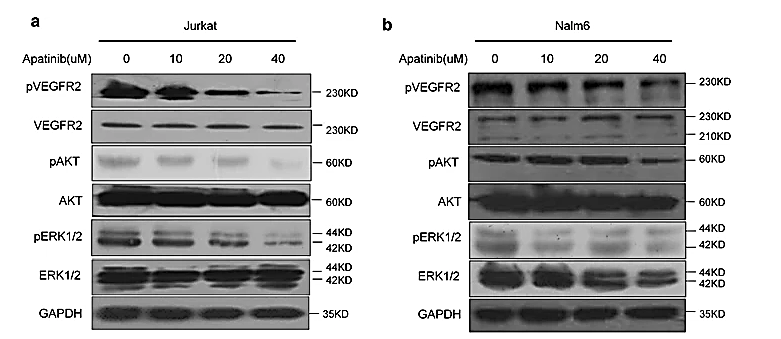
Figure 10: Apatinib inhibited phosphorylation of VEGFR2 and the subsequent signalling pathway as it was applied to Jurkat (a) and Nalm6 (b) cell lines of ALLs, Treatment duration: 48 hours; Concentration (in μM): 0, 10, 20, 40.
As a potential therapy plan, the best dosage of Apatinib for treating various cancer types is yet to be discovered via further research and during prolonged drug surveillance. To choose the best candidates for anti-angiogenesis medicines based on big data, it is important to discover predictive biomarkers.













Kommentare