In many areas of biomedical research, such as drug development, environmental toxicology, and disease pathology, cellular toxicity is an essential metric. Researchers frequently rely on trustworthy assays to precisely quantify cytotoxicity, and one such technique that is becoming increasingly popular is the LDH (lactate dehydrogenase) assay. LDH is a reliable marker of cytotoxicity since it is a stable cytoplasmic enzyme that is released when a cell is damaged or dies. The oxidoreductase lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate and lactate. Cytotoxic substances frequently cause necrosis or apoptosis, which compromises the integrity of the cell membrane. When a membrane is damaged, the stable cytosolic enzyme LDH is released into the surrounding cell milieu. As a result, LDH is frequently assessed to determine whether tissue or cell damage is present. The stucture of LDH is shown in Figure 1.
Figure 1: The structure of LDH.
Measuring the amount of markers that seep into the culture media from the cytoplasm can reveal the existence of dead cells that have lost their membrane integrity. When conducting this kind of test, lactate dehydrogenase is the most often employed marker. Pyruvate is converted to lactate by lactate dehydrogenase (LDH), which also catalyzes the conversion of NAD+ to NADH. Numerous substrate molecules can be reduced by NADH's reducing ability to produce colorful, fluorescent, or luminogenic products . The overall design and test chemistry for detecting LDH-release from dead cell cytoplasm are shown in Figure 2. In a reagent mixture, an excess of lactate and NAD+ are supplied as substrates to stimulate LDH to produce pyruvate and NADH. Resazurin, the substrate, is changed into resorufin, the fluorogenic product, using NADH's reducing power. The diaphorase substrate in colorimetric variants of this assay chemical is a tetrazolium molecule, which is transformed into a highly colored formazan product that can be detected using a spectrophotometer.
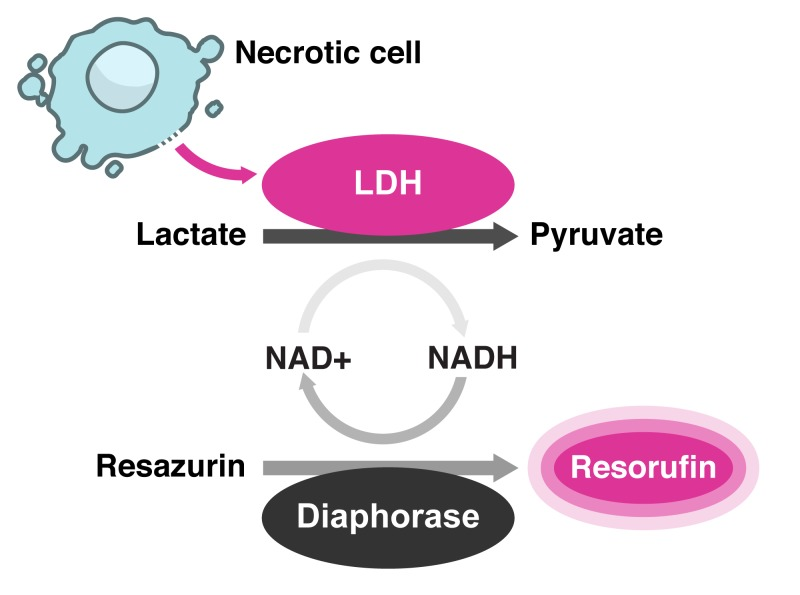
Figure 2: A schematic representation of the fluorogenic LDH-release assay chemistry.
A schematic representation of the fluorogenic LDH-release assay chemistry.
colorimetric variants of this assay chemical is a tetrazolium molecule, which is transformed into a highly colored formazan product that can be detected using a spectrophotometer .
In biological research, analyzing the impact on suppression of cell growth and/or cell death has been crucial. This objective is most typically served by the LDH-based cytotoxicity test. The initial procedure for LDH-based cytotoxicity significantly underestimated the percentage of dead cells in growth-inhibited circumstances. Here a straightforward modified LDH-based cytotoxicity procedure that adds more condition-specific controls in order to get over the limitation. Thus, in addition to measuring overall impacts, this updated methodology can also assess killing effects more accurately, especially in settings where growth inhibition is present. However, many therapeutic agents need to undergo multiple replication cycles before a killing effect becomes apparent, and when employing an LDH-based cytotoxicity assay with current protocols, dose-dependent growth inhibition can act as a confounding variable, leading to a gross underestimation of the number of dead cells. This is a modified technique for an assay that uses a straightforward absorbance plate reader and just one 96-well format to more accurately determine the overall effect, the percentage of growth inhibition, and the percentage of death . This procedure is meant to be used with the cytotoxicity detection kit . By including condition-specific controls, it makes it possible to utilize this kit to assess cellular death, particularly in investigations that need relatively long treatment times or considerable cell cycle inhibition. The percentage of growth inhibition, total effect, and percentage of cell death can all be found in a single experiment using this modified protocol .
You can utilize an LDH-based assay to find out what proportion of cells are dead in a specific situation. Nevertheless, the LDH-based assay might significantly underestimate the proportion of dead cells when there is growth inhibition present, as is the case with PLX4720 therapy (Figure 3). This is so because the conventional methodology uses a single control in which Triton X-100 is introduced to the culture to induce all of the cells to break down as a basis for the complete probable release of LDH. This is carried out on the vehicle in its treated state. The total possible amount of LDH is then determined using the supernatant from these wells as a reference .
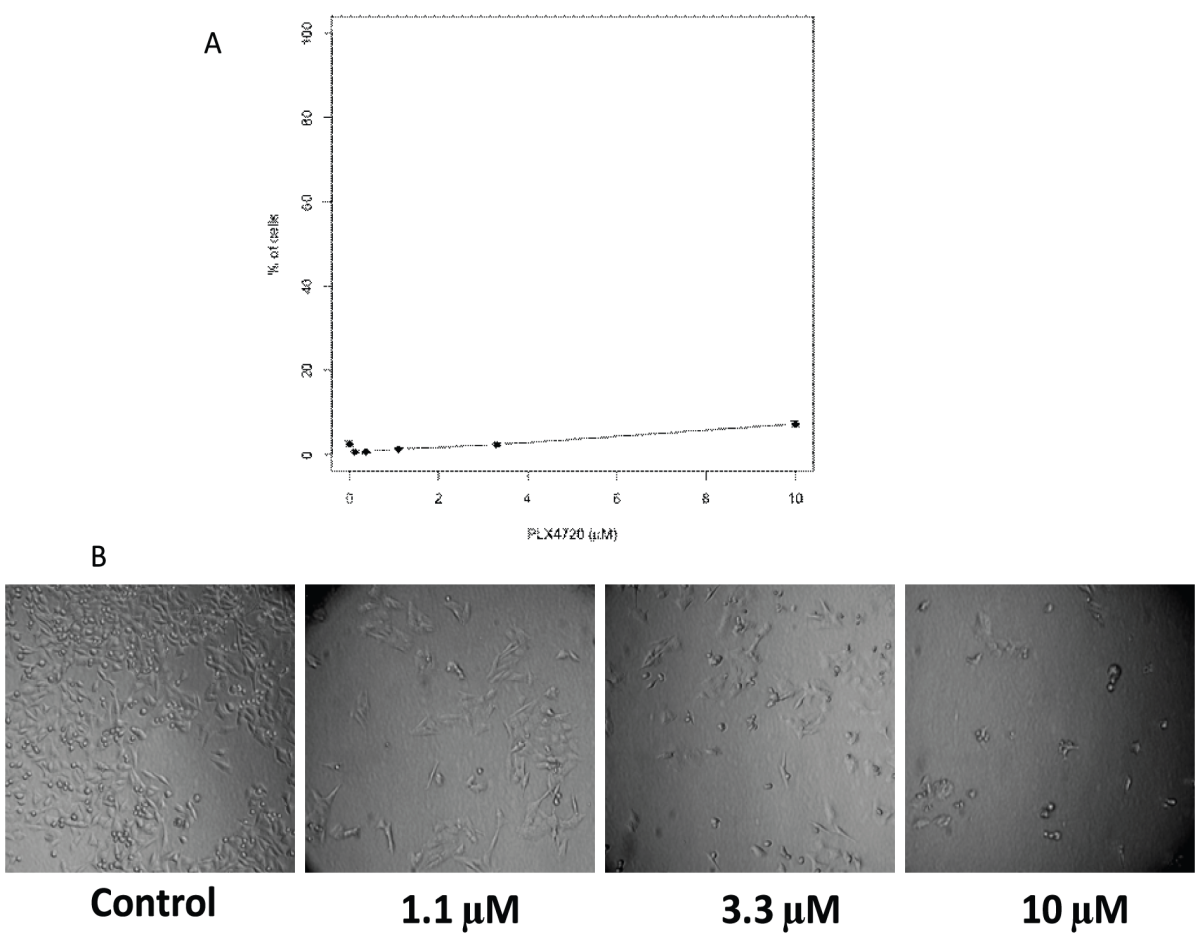
Figure 3: Original cytotoxicity protocol grossly underestimated the proportion of dead cells in conditions with growth inhibition.
The controls for the DMSO control, which had much more cells and so contained higher quantities of total LDH than that in 10 µM treated conditions, were used to determine the maximum quantity of LDH that could be produced using the usual methodology. One simple solution to this issue is to add samples to be lysed by Triton X-100 as the high control for each condition in the standard process, thereby using condition-specific controls for each condition. This would make it possible to calculate the proportion of destroyed cells under conditions even in the presence of considerable inhibition of cell growth. The differences between the modified protocol and the conventional protocol are shown in Figure 4.
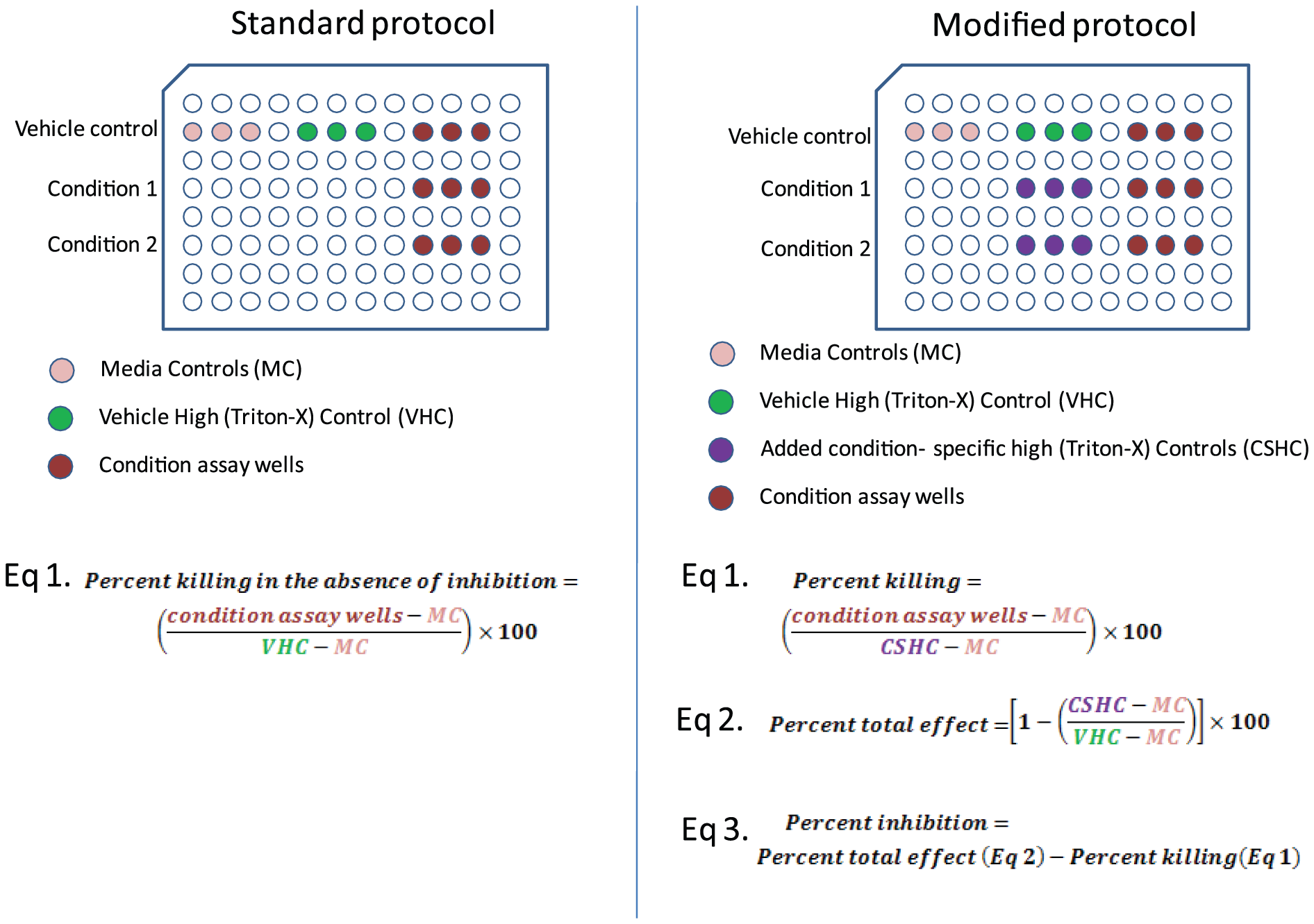
Figure 4: Comparison of experimental layout between the standard and modified protocols.
The overall impact of a particular condition can also be determined using the total LDH controls (Figure 4). The percentage of effective growth inhibition can be obtained by deducting the percentage of dead by the new approach from the overall affect. Consequently, we discover that growth inhibition accounted for 46% of the impact in the 10 µM condition. As a result, you can ascertain the overall effect of a treatment or condition as well as the percentage of cells killed and inhibited with just one experiment.
The half-life of LDH that is released from cells into the surrounding media is roughly nine hours, however it should be verified for each cell culture model that is being used as it may vary depending on the cause of cell death. The amount of active LDH left in the culture media at the end of the experiment may be underestimated compared to the amount of LDH present in untreated cells if treated cells die (or if Lysis Solution is added) at the start of an experimental exposure period . It is advised to fully lyse a set of control wells at the start and finish of the incubation period. The rate of cell proliferation throughout the experiment is another issue to take into account. Untreated control wells will have more cells and, thus, more LDH activity at the end of the exposure period if the indicator cells are growing throughout the experiment. Ham's F12, Iscove's, and various DMEM formulations are examples of pyruvate-supplemented media that may reduce the fluorescence signal because they suppress the LDH enzymatic process, which catalyzes the conversion of lactate to pyruvate . It might be difficult to measure the mortality of a particular cell population when multiple cell types are present. The spontaneous release of LDH from dying effector cells must be taken into consideration, even though LDH-release has been employed to identify target cell killing in mixtures of target and effector cells. When several effector to target cell ratios are employed in research, this might be particularly difficult to implement .
To sum up, the LDH assay is a useful tool for scientists looking to evaluate cytotoxicity in a range of experimental contexts. It is a recommended approach for assessing how chemicals affect cell viability because of its sensitivity, simplicity of use, and quantitative character. LDH tests are probably going to stay a mainstay in the study of cytotoxicity as biomedical research develops, adding to our knowledge of medication safety, environmental effects, and disease causes.














Kommentare