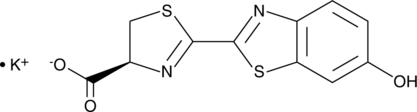
D-Luciferin (Potassium Salt)
D-luciferin Potassium salt, commonly known as K-luciferin, is a chemical molecule that is essential in the field of bioluminescence. Luciferin K, derived from the Latin word "lucifer" meaning "light-bringing," is a vital component in the emission of light by diverse organisms such as fireflies, glow worms, and deep-sea critters. Because of its potential to produce a highly sensitive and precise light signal, Luciferin K has received a lot of attention in scientific studies and various applications. The 2D molecular structure of D-Luciferin K is depicted in Figure 1.
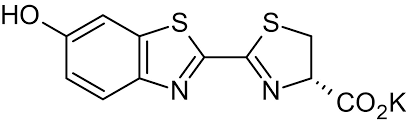
Figure 1: The molecular structure of D-luciferin K.
The chemical creation of light by a living creature is known as bioluminescence. Luciferase is the enzyme involved in bioluminescent reactions. The various substrates are referred to as luciferins. The luciferins and oxygen undergo a chemical reaction that luciferase aids in catalyzing, or accelerating. The luciferin molecule is oxidized during this chemical reaction, producing light and the inactive oxyluciferin molecule. As long as luciferin and oxygen are present, luciferase can continue to emit light in the form of bioluminescence after the chemical reaction as shown in Figure 2.
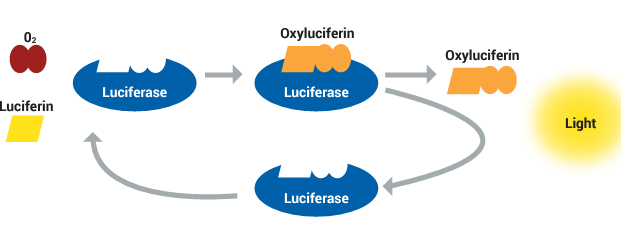
Figure 2: Chemical reaction leading to bioluminescence.
Approximately 40 different species of firefly, click-beetles, and rail-road worms contain the same substrate, D-luciferin, even though each species of bioluminescent insect has its own unique luciferase enzyme . The oxidation of D-luciferin 1 is catalyzed by the 62 kDa insect luciferase (Fluc) in two different steps in the bioluminescence reaction: first, by adenylation, which activates the carboxyl group of 1, and then, by oxidation of the luciferyl-adenylate 2, which forms oxyluciferin 4 as an excited state anionic species through a dioxetanone intermediate 3 (Figure 3). By emitting a light photon, the excited state of oxyluciferin species 4 relaxes to its ground state 5 . The bioluminescence reaction's quantum yield is limited when 20% of luciferyl adenylate undergoes a dark-side oxidation that produces H2O2 and dehydro-luciferyladenylate 6, a potent luciferase inhibitor (Ki = 3.8 ± 0.7 nM). In addition to being protonated 5, oxyluciferin inhibits the luciferase enzyme (Ki = 500 ± 30 nM). Firefly luciferase catalyzes light emission from D-luciferin with a maximum quantum yield of 41% at pH = 8.5, owing to the dark-side reaction and additional energy losses .
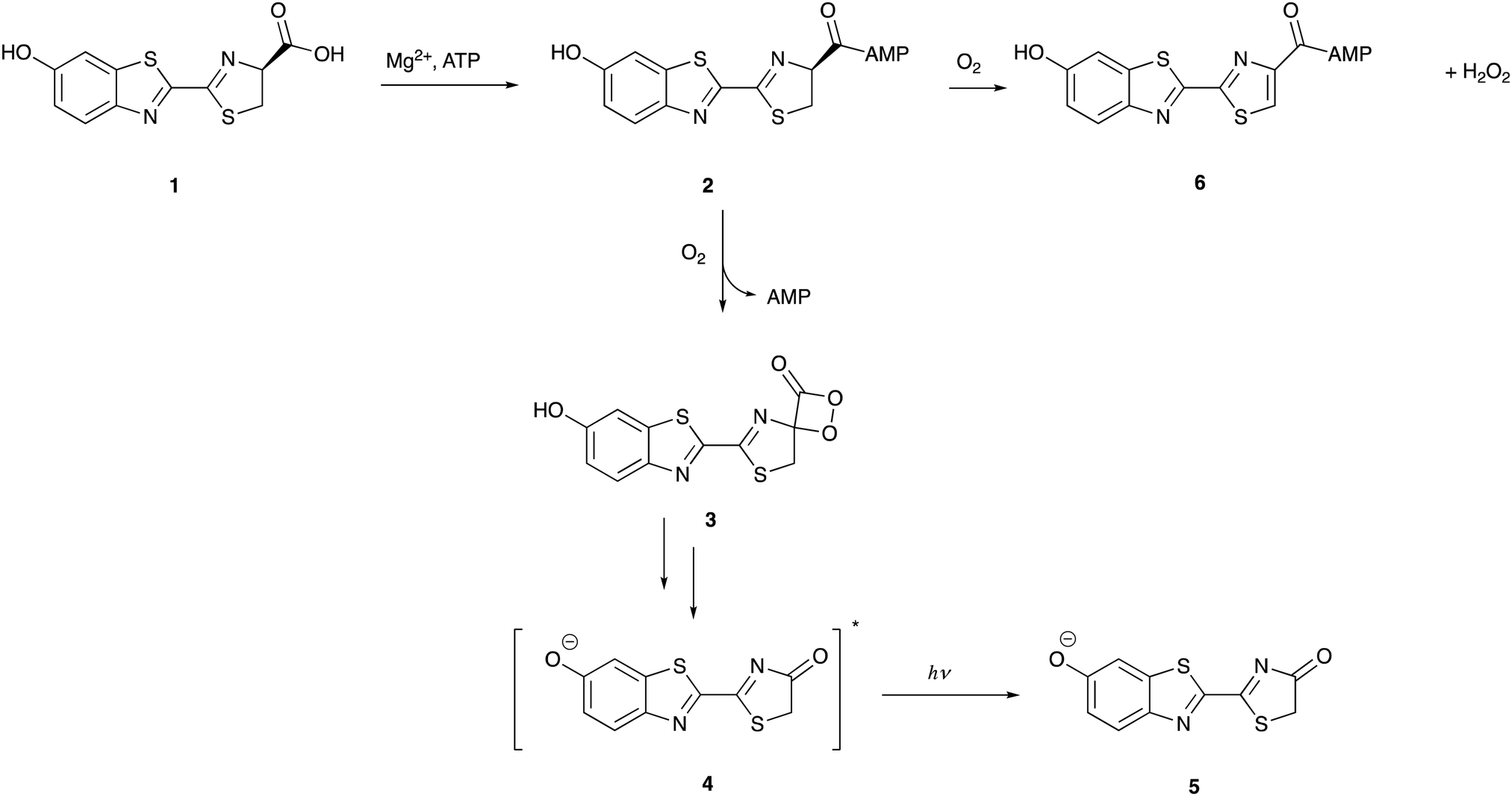
Figure 3: The mechanism of firefly bioluminescence
One distinct characteristic of D-luciferin bioluminescence that is not shared by other luciferins, such as coelenterazine, is the activation of D-luciferin by ATP prior to oxidation. D-luciferin is more stable in solution and less prone to auto-oxidation, it requires adenylation to activate it, which reduces background chemiluminescence. Almost all eukaryotic and prokaryotic cells have ATP, which is referred to as the "energy currency" of a cell. ATP levels vary widely among these organisms. The D-luciferin/Fluc test has been widely adapted to assess ATP concentration in the double digit nanomolar area in many systems for both quality control and hygiene, frequently to determine the level of bacterial contamination. This is because the assay produces an ATP-dependent light output, Additionally, it is employed to track reactions that produce and degrade ATP .
D-luciferin is reported to be less poisonous and more soluble in water than coelenterazine. Additionally, D-luciferin has the longest emission wavelength (λmax = 558 nm39) and the most impressive quantum yield (41.0 ± 7.4%) among all known luciferin/luciferase systems (compared to 15–30% for most luciferase/luciferin pairs). These characteristics make it more suitable for imaging applications involving blood (P. S. F. McCapra et al., 1994). Tissues and hemoglobin absorb light most powerfully at wavelengths less than 500 nm. It is possible to genetically encode the luciferase gene of interest in mammalian cells as well as other biological entities of interest, such as bacteria, fungi, viruses, and protozoa, and then introduce them into a small mammal for purposes of proliferation, monitoring, and imaging. The most often utilized pair of naturally occurring luciferins and luciferases for in vivo imaging is D-luciferin and firefly luciferase from the North American firefly, Photinus pyralis as shown in Table 1.
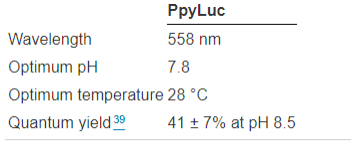
Table 1: Characteristics of the American firefly Photinus Pyralis's firefly luciferase.
Blood and tissue nevertheless absorb a significant amount of D-luciferin bioluminescence light, despite it being red-shifted in comparison to other naturally occurring luciferins. Light in the near infrared (650–900 nm) penetrates blood and tissue more easily. Although some light falls into this desired range of wavelengths in the broad emission spectrum of D-luciferin, additional red-shifted light would enable more sensitive imaging .
D-luciferin is not the best option for bioluminescence imaging due to other variables. For instance, in vivo studies involving D-luciferin require high dosages due to its low cell permeability. The heterogeneous bio-distribution of the radioactive D-luciferin substrate in rats has also been shown in studies employing the 14C-labelled radioactive substrate . Furthermore, the probe is not well absorbed by certain target organs, including the brain. While D-luciferin can emit light at different wavelengths when it interacts with different Fluc mutants, this range of wavelengths does not make it suitable for in vivo multi-parametric imaging, especially in deep tissue imaging, as the most red-shifted λmax that can be obtained by combining Fluc mutants with D-luciferin is 620 nm . This wavelength of light is absorbed, attenuated, and scattered in tissues, which makes spectral unmixing from various luciferases more difficult. The design, production, and testing of novel D-luciferin analogues have helped to overcome some of these obstacles, and in certain circumstances, this has resulted in brighter, red-shifted emission.
Alot of work has been done in the field of protein engineering to produce mutant firefly luciferases with improved proteolytic, thermostability, and pH stability. It is true that even a minor alteration to the enzyme's sequence can have a big impact on the substance and emission of the enzyme. As illustrated in Figures A and B, the Japanese Firefly Luciferase mutant S286N exhibits red-shifted emission at λmax = 605 nm, in contrast to the wild-type at λmax = 560 nm. This is due to the fact that the mutation of Ser 286 to an asparagine residue disrupts a crucial hydrogen-bonding network in which it is implicated . Additionally, this results in a modification of Ile 288's conformation near the emitter, increasing the hydrophobic pocket's flexibility (Figs. C and D).
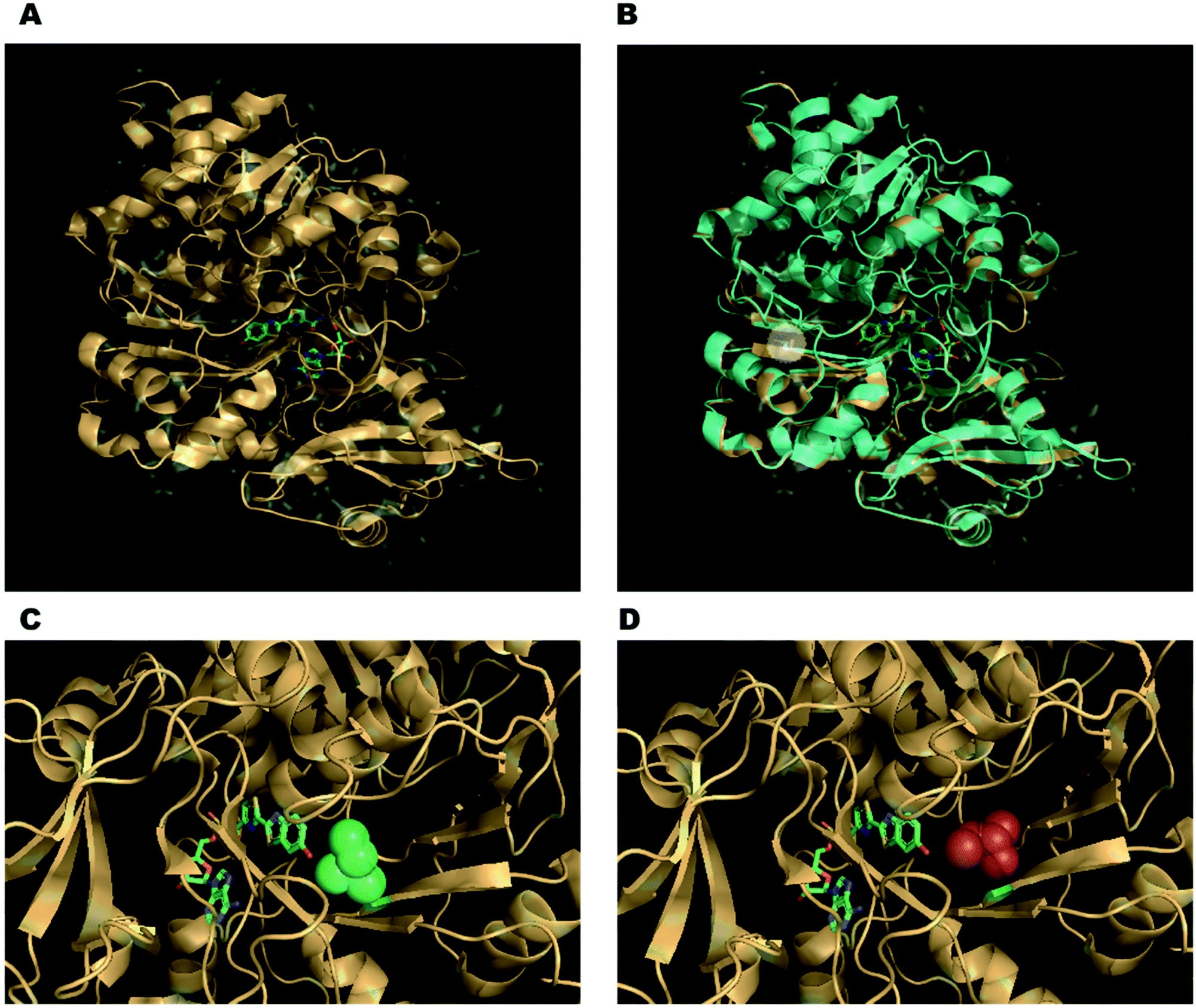
Figure 5: (A) 5′-O-[N-(dehydroluciferyl)-sulfamoyl]adenosine (DLSA), a high-energy intermediate analogue of the Japanese firefly luciferase, co-crystal structure. (B) Luciola cruciata luciferase complex with DLSA superimposed on top of mutant S286N complex with DLSA. The residues of the critical mutation's side chains are represented as sticks and are emphasized in the white circle. (C) The Ile 288 conformation in wild-type Luciola cruciata, which consists of green spheres. (D) The S286N mutant's altered Ile 288 conformation, which results in a more flexible hydrophobic pocket.
D-luciferin, is an extraordinary chemical that has expanded our knowledge of the natural world. In the years to come, as we continue to explore the secrets surrounding bioluminescence, it's possible that we'll discover even more incredible uses for this extraordinary molecule, which will illuminate the wonders of both human ingenuity and the natural world.










Kommentare