O-Acetyl Salicylhydroxamic Acid (Synonyms: AcSHA,O-ASHA) |
| Katalog-Nr.GC14999 |
irreversible, non-selective inhibitor of COX-1 and COX-2
Products are for research use only. Not for human use. We do not sell to patients.
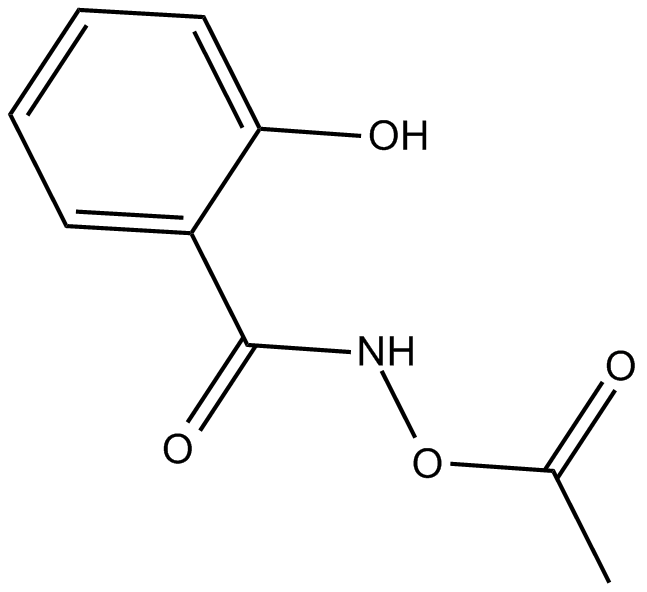
Cas No.: 199854-00-7
Sample solution is provided at 25 µL, 10mM.
O-Acetyl Salicylhydroxamic Acid (O-ASHA) is an irreversible, non-selective inhibitor of COX-1 and COX-2 [1].
Cyclooxygenase (COX) is the key enzyme required for the conversion of arachidonic acid to prostaglandins. Cyclooxygenase enzymes have been involved in diverse physiological situations and disease processes ranging from inflammation to cancer. Until now, two cyclooxygenase isoforms have been identified, COX-1 and COX-2. The COX-1 enzyme is produced constitutively (i.e., gastric mucosa) and COX-2 is inducible (i.e., sites of inflammation) [2].
O-Acetyl Salicylhydroxamic Acid (O-ASHA) inhibited the activity of ovine COX-1 in a time-dependent and irreversible manner with a 50% B/B0 value of approximately 4.5 mM [1]. O-Acetyl Salicylhydroxamic Acid was a novel acetylating agent. O-Acetyl Salicylhydroxamic Acid inhibited PGE2 synthesis in vivo and blocked the cyclooxygenase activity of PGHS in vitro. O-Acetyl Salicylhydroxamic Acid elicited its effects via acetylation of Ser-529 in the cyclooxygenase active site [1].
References:
[1] Loll P J, Sharkey C T, O'Connor S J, et al. O-acetylsalicylhydroxamic acid, a novel acetylating inhibitor of prostaglandin H2 synthase: structural and functional characterization of enzyme-inhibitor interactions[J]. Molecular pharmacology, 2001, 60(6): 1407-1413.
[2] Dubois R N, Abramson S B, Crofford L, et al. Cyclooxygenase in biology and disease[J]. The FASEB journal, 1998, 12(12): 1063-1073.
Average Rating: 5 (Based on Reviews and 1 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















