Ibafloxacine (R835) |
| Katalog-Nr.GC33988 |
Ibafloxacin (R835) (R835) ist ein Fluorchinolon-Antibiotikum, das ausschließlich fÜr den veterinÄrmedizinischen Gebrauch entwickelt wurde.
Products are for research use only. Not for human use. We do not sell to patients.
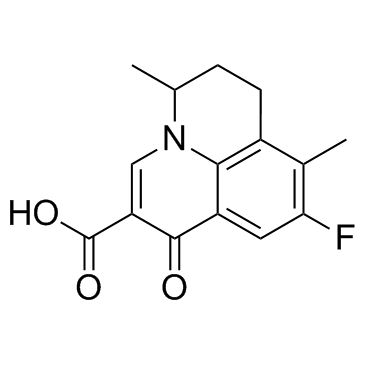
Cas No.: 91618-36-9
Sample solution is provided at 25 µL, 10mM.
Ibafloxacine (R835) is a fluoroquinolone antibiotic agent that is developed exclusively for veterinary use.
The pharmacokinetic behavior of Ibafloxacin is studied after intravenous administration of a single dose of 15 mg/kg to 6 healthy lactating goats. Plasma concentrations of Ibafloxacin are determined by high-performance liquid chromatography with fluorescence detection. After IV injection Ibafloxacin shows very rapid initial distribution, with a mean half-life of 0.35 h, follows by slower elimination, with a mean half-life of 3.76 h. The elimination half-life of Ibafloxacin after oral administration has been reported to be 3.83 h in dogs and 3.00 h in cats[1].
[1]. Marín P, et al. Pharmacokinetics and milk penetration of ibafloxacin after intravenous administration to lactating goats. Can J Vet Res. 2007 Jan;71(1):74-6.
Average Rating: 5 (Based on Reviews and 27 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















