Ox Stress Reagents
- Antioxidants(219)
- Carbohydrate Oxidation(3)
- Free Radical Generators(4)
- Hydrogen Sulfide Donors(5)
- Lipid Peroxidation(168)
- NO Donors(17)
- Pro-Oxidant Activity(42)
- Protein Oxidation(13)
- Spin Traps(9)
Products for Ox Stress Reagents
- Cat.No. Nom du produit Informations
-
GC11123
α-CEHC
A major metabolite of δ-tocopherol

-
GC52253
α-Enolase (1-19)-biotin Peptide
A biotinylated α-enolase peptide

-
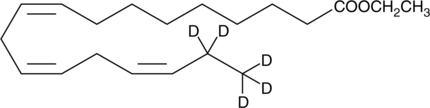
GC45601
α-Linolenic Acid ethyl ester-d5
-
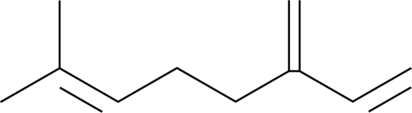
GC41502
β-Myrcene
β-Myrcène (β-β-Myrcène), un composé volatil aromatique, supprime l'activité NF-κB induite par le TNFα.
-
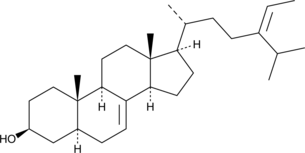
GC48317
δ7-Avenasterol
A phytosterol that has antioxidant activities
-
GC45713
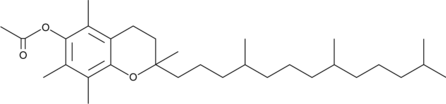
(±)-α-Tocopherol Acetate
(±)-α-acétate de tocophérol ((±)-acétate de vitamine E), est une forme synthétique active par voie orale de la vitamine E.
-
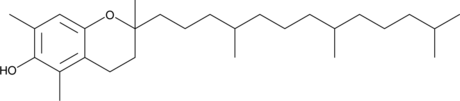
GC40015
(±)-5,7-Dimethyltocol
(±)-5,7-Dimethyltocol is a form of tocopherol.
-
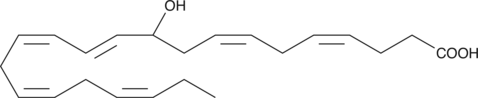
GC41213
(±)10-HDHA
(±)10-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC41214
(±)11-HDHA
(±)11-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
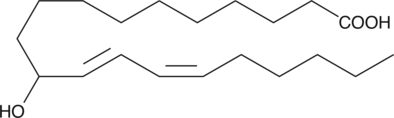
GC40428
(±)11-HEDE
(±)11-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.
-
GC40467
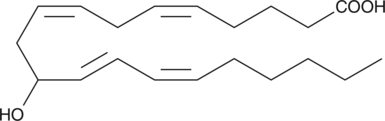
(±)11-HETE
(±)11-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC40359
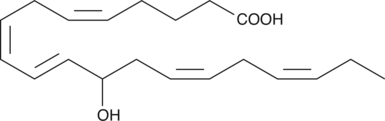
(±)12-HEPE
(±)12-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC40429
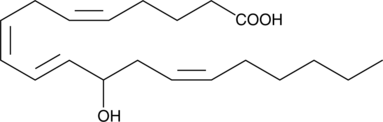
(±)12-HETE
(±)12-HETE, un produit métabolique majeur de l'acide arachidonique utilisant la catalyse 12-LOX, inhibe l'apoptose cellulaire de manière dose-dépendante.
-
GC41192
(±)13-HDHA
(±)13-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
GC41649
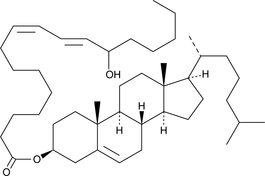
(±)13-HODE cholesteryl ester
(±)13-HODE cholesteryl ester was originally extracted from atherosclerotic lesions and shown to be produced by Cu2+-catalyzed oxidation of LDL.
-
GC40355
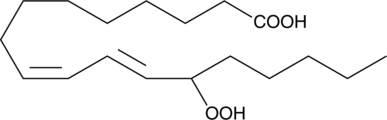
(±)13-HpODE
(±)13-HpODE (acide 13-hydroperoxylinoléique) est un mélange racémique d'hydroperoxydes, qui est produit par l'oxydation de l'acide linoléique par la lipoxygénase.
-
GC41193
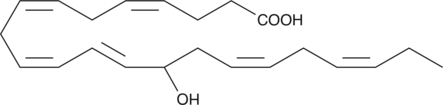
(±)14-HDHA
(±)14-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC40420
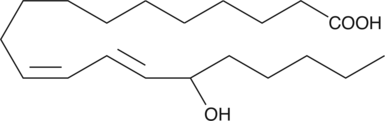
(±)15-HEDE
(±)15-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.
-
GC40361
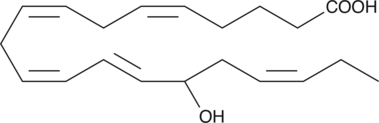
(±)15-HEPE
(±)15-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC41196
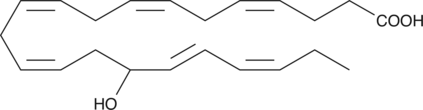
(±)16-HDHA
(±)16-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC41197
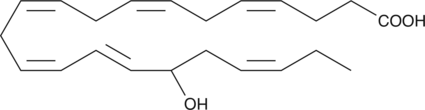
(±)17-HDHA
(±)17-HDHA is an autoxidation product of docosahexaenoic acid in vitro.
-
GC40362
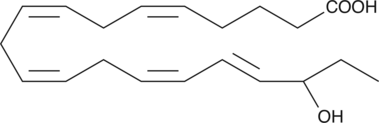
(±)18-HEPE
(±)18-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC41202
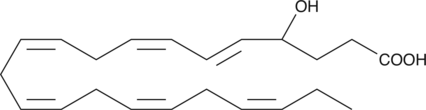
(±)4-HDHA
(±)4-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC40364
(±)5-HEPE
(±)5-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC40439
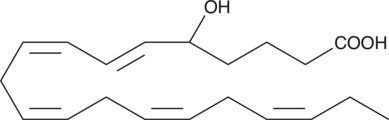
(±)5-HETE
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC40828
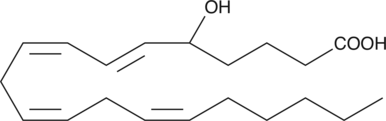
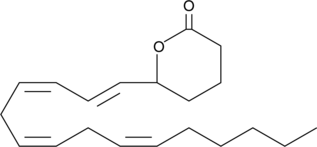
(±)5-HETE lactone
(±)5-HETE lactone is a cyclic ester formed by acid-catalyzed nucleophilic addition of the C-5 hydroxyl to the C-1 carboxyl of (±)5-HETE.
-
GC40837
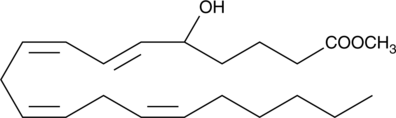
(±)5-HETE methyl ester
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
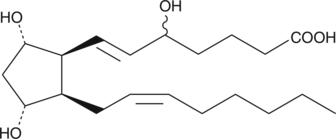
GC40576
(±)5-iPF2α-VI
Isoprostanes are prostaglandin (PG)-like products of free-radical induced lipid peroxidation.
-
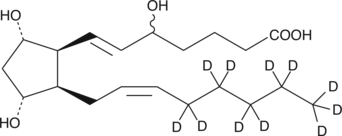
GC46263
(±)5-iPF2α-VI-d11
An internal standard for the quantification of (±)5iPF2αVI
-
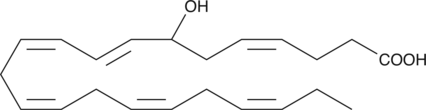
GC41204
(±)7-HDHA
(±)7-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
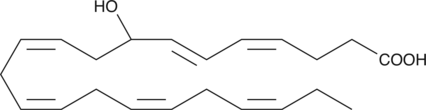
GC41205
(±)8-HDHA
(±)8-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
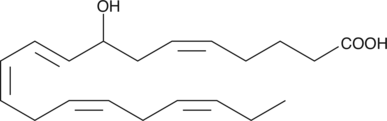
GC40366
(±)8-HEPE
(±)8-HEPE is produced by non-enzymatic oxidation of EPA.
-
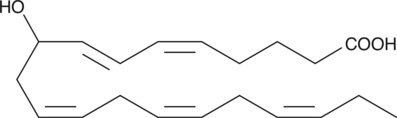
GC40367
(±)9-HEPE
(±)9-HEPE is produced by non-enzymatic oxidation of EPA.
-
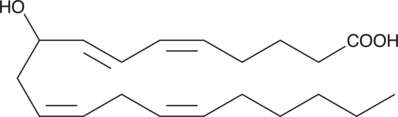
GC40443
(±)9-HETE
(±)9-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
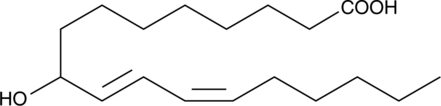
GC40541
(±)9-HODE
(±)9-HODE is one of the two racemic monohydroxy fatty acids resulting from the non-enzymatic oxidation of linoleic acid.
-
GC40356
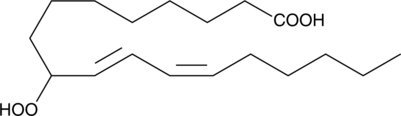
(±)9-HpODE
(±)9-HpODE est un hydroperoxyde lipidique à longue chaîne, est un produit de la peroxydation de l'acide linoléique.
-
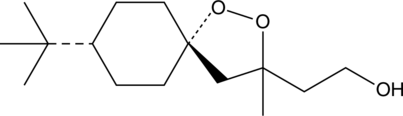
GC45248
(-)-FINO2
Le (-)-FINO2 est un puissant inducteur de la ferroptose. (-)-FINO2 inhibe l'activité de GPX4. (-)-FINO2 est un oxydant stable qui oxyde le fer ferreux et stable À différents niveaux de pH. (-)-FINO2 provoque une peroxydation lipidique généralisée.
-
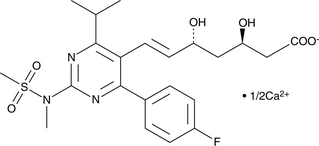
GC49690
(3R,5R)-Rosuvastatin (calcium salt)
A potential impurity found in bulk preparations of rosuvastatin
-
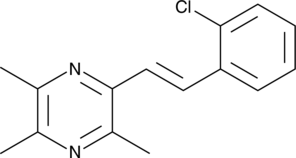
GC41700
(E)-2-(2-Chlorostyryl)-3,5,6-trimethylpyrazine
(E)-2-(2-Chlorostyryl)-3,5,6-trimethylpyrazine (CSTMP) is a stilbene derivative with antioxidant and anticancer activities.
-
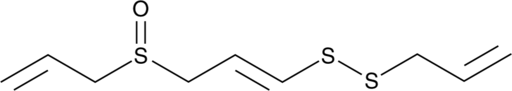
GC49003
(E)-Ajoene
A disulfide with diverse biological activities
-
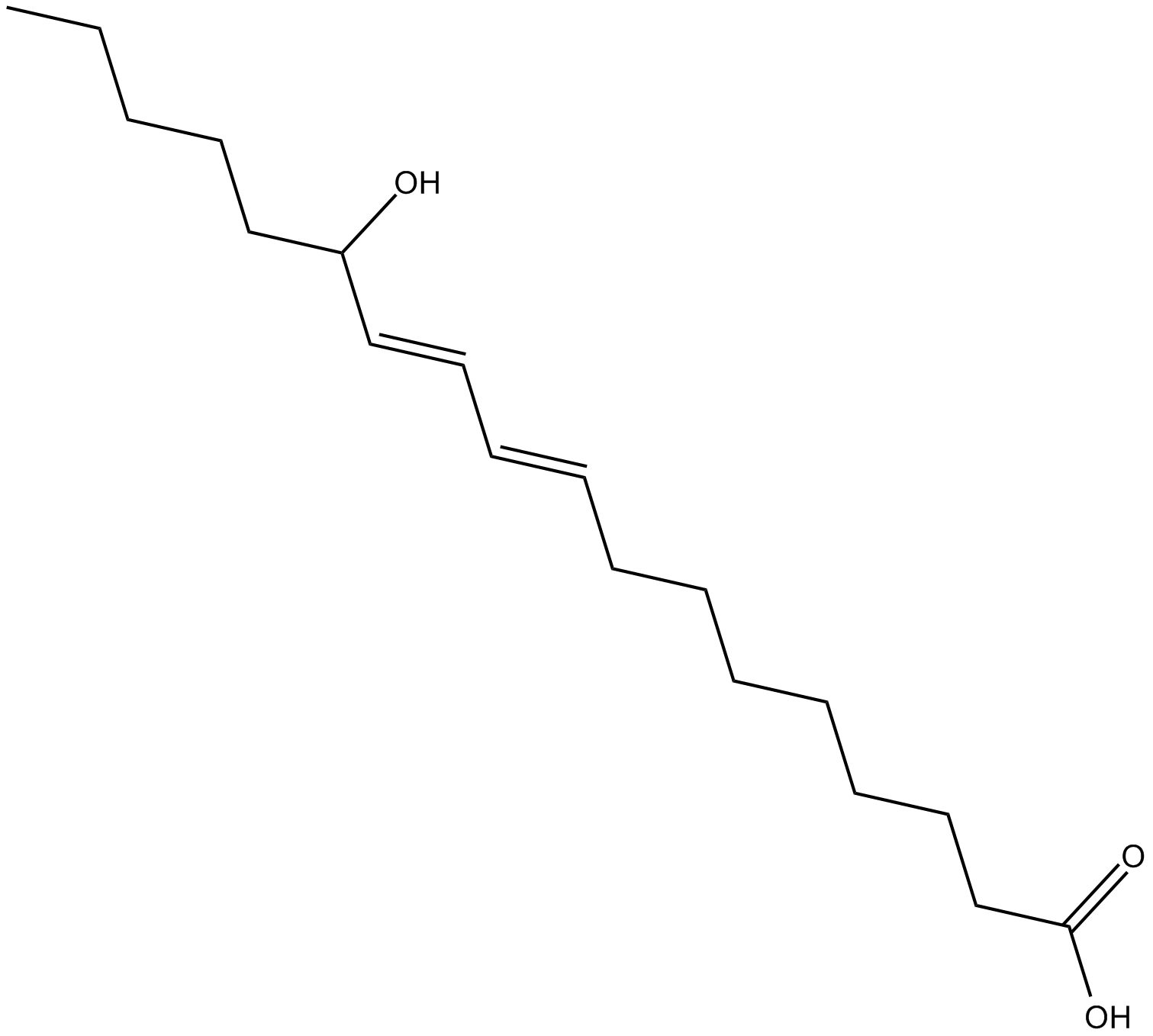
GC19461
(±)13-HODE
(±)13-HODE is one of the two racemic monohydroxy fatty acids resulting from the non-enzymatic oxidation of linoleic acid.
-
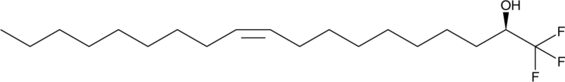
GC49034
1(R)-(Trifluoromethyl)oleyl alcohol
An oleic acid analog
-
GC41837
1,3,7-Trimethyluric Acid
L'acide 1,3,7-triméthylurique est le métabolite de la caféine. Le rapport métabolique de l'acide 1,3,7-triméthylurique À la caféine peut être évalué en tant que biomarqueur pour décrire la variabilité de l'activité du CYP3A dans une cohorte.

-
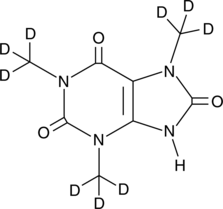
GC46387
1,3,7-Trimethyluric Acid-d9
An internal standard for the quantification of 1,3,7-trimethyluric acid
-
GC46481
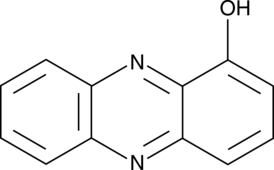
1-Hydroxyphenazine
A P. aeruginosa virulence factor
-
GC18235
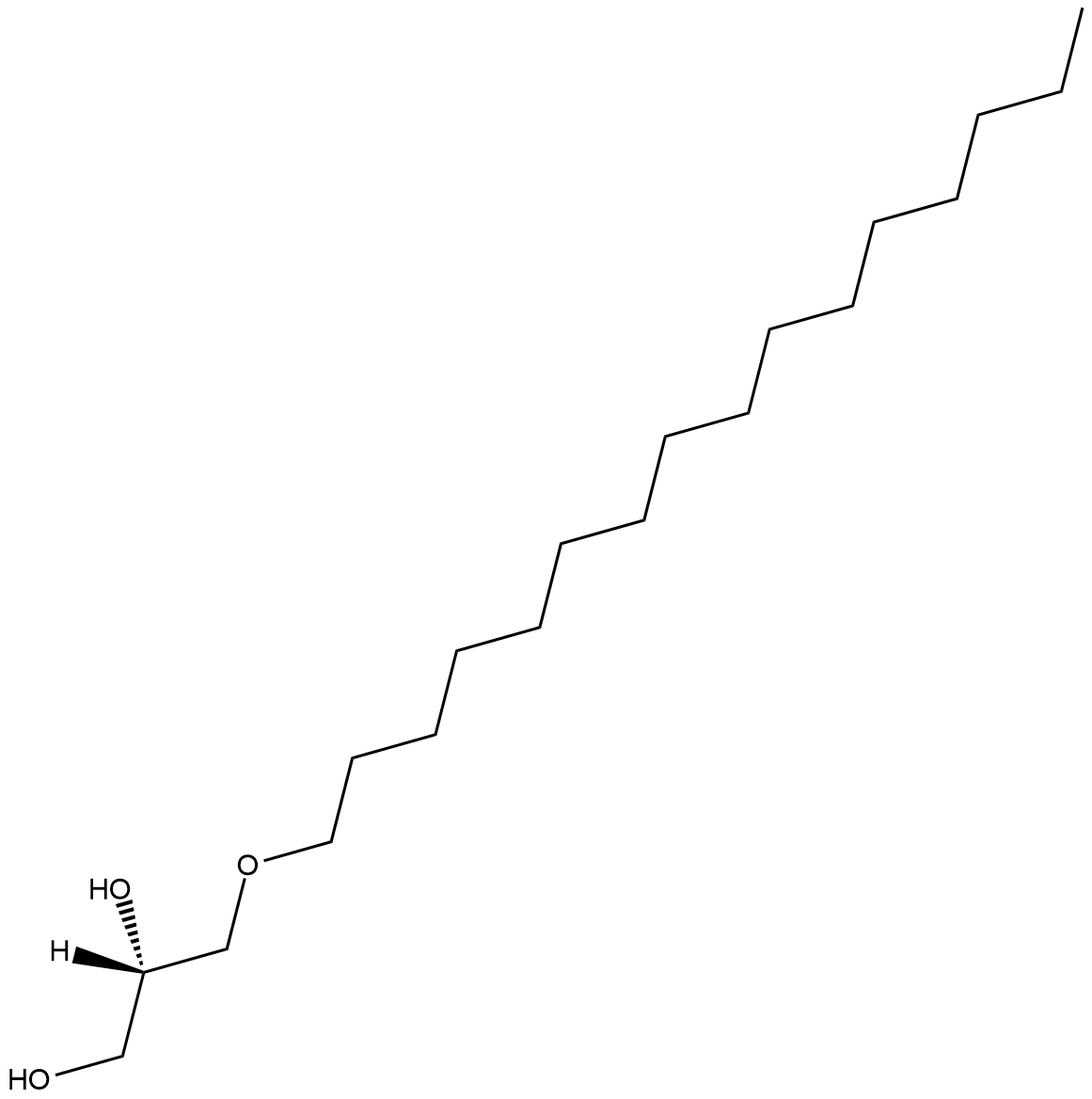
1-O-Hexadecyl-sn-glycerol
1-O-Hexadecyl-sn-glycerol is a bioactive alkyl glyceryl ether.
-
GC40146
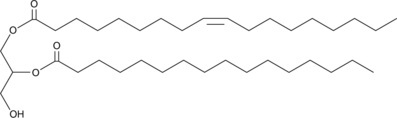
1-Oleoyl-2-Palmitoyl-rac-glycerol
1-Oleoyl-2-palmitoyl-rac-glycerol (1,2-OP) is a diacylglycerol containing oleic acid at the sn-1 position and palmitic acid at the sn-2 position.
-
GC42026
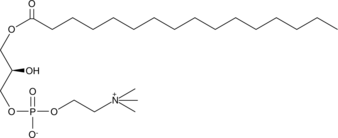
1-Palmitoyl-2-hydroxy-sn-glycero-3-PC
Le 1-palmitoyl-2-hydroxy-sn-glycéro-3-PC est un LPC gonadique abondant (lysophosphatidylcholine).
-
GC45693
1-Palmitoyl-d9-2-hydroxy-sn-glycero-3-PC
A quantitative analytical standard guaranteed to meet MaxSpec® identity, purity, stability, and concentration specifications
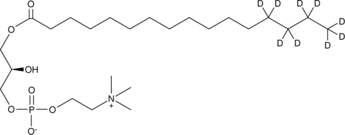
-
GC48782
10,13-epoxy-11-methyl-Octadecadienoic Acid
A furan fatty acid
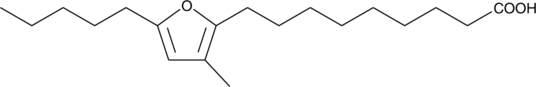
-
GC41866
10-methyl-9-(phenoxycarbonyl) Acridinium (trifluoromethylsulfonate)
10-methyl-9-(phenoxycarbonyl) Acridinium is an acridinium ester that produces fluorescent 10-methyl-9-acridone upon oxidation with hydrogen peroxide, persulfates, and other oxidants in alkaline conditions.
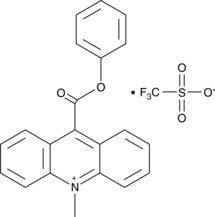
-
GC41868
10-Nitrooleate
Le 10-nitrooléate (CXA-10), un acide gras nitré, a des effets potentiels dans les états pathologiques dans lesquels le stress oxydatif, l'inflammation, la fibrose et/ou la toxicité tissulaire directe jouent un rÔle important.
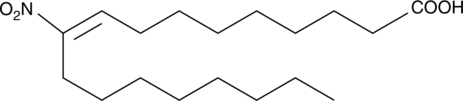
-
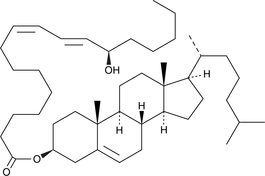
GC41893
13(R)-HODE cholesteryl ester
13(R)-HODE cholesteryl ester was originally extracted from atherosclerotic lesions.
-
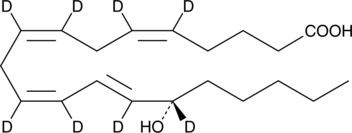
GC46442
15(S)-HETE-d8
An internal standard for the quantification of 15-HETE
-
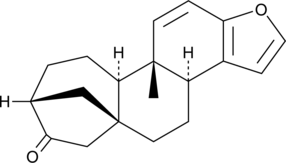
GC46452
16-Oxokahweol
A synthetic diterpene
-
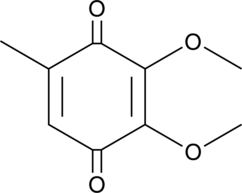
GC40947
2,3-Dimethoxy-5-methyl-p-benzoquinone
La 2,3-diméthoxy-5-méthyl-p-benzoquinone (CoQ0) est un puissant composé d'ubiquinone actif oral qui peut être dérivé d'Antrodia cinnamomea.
-
GC40416
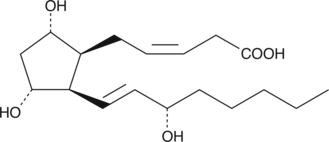
2,3-dinor-8-iso Prostaglandin F2α
8-iso Prostaglandin F2α (8-iso PGF2α; 8-isoprostane) is a prostaglandin-like product of non-specific lipid peroxidation.
-
GC46057
2,5-Dihydroxycinnamic Acid phenethyl ester
An inhibitor of 5-LO
-
GC40503
2-HOBA
Le 2-HOBA (2-HOBA), un piégeur sélectif de dicarbonyle, est un antioxydant et un piégeur de radicaux libres et d'isolevuglandines (IsoLG).
-
GC49172
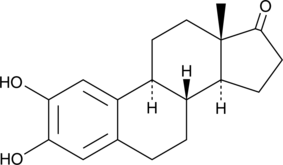
2-hydroxy Estrone
La 2-hydroxyestrone (catécholestrone) est un agent anti-œstrogénique médié par des récepteurs spécifiques.
-
GC49840
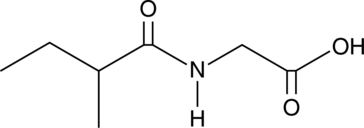
2-Methylbutyrylglycine
A metabolite of isoleucine
-
GC42195
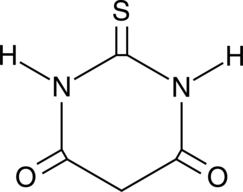
2-Thiobarbituric Acid
2-Thiobarbituric acid is a colorimetric reagent commonly used in the detection of malondialdehyde (MDA), a marker of lipid peroxidation.
-
GC41210
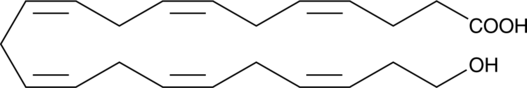
22-HDHA
22-HDHA is an oxidation product of docosahexaenoic acid.
-
GC49005
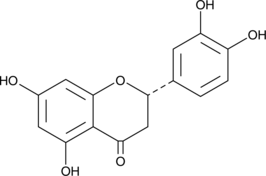
2S-Eriodictyol
A flavanone with antioxidant activity
-
GC40618
3',4',7-Trihydroxyisoflavone
3',4',7-Trihydroxyisoflavone, un métabolite majeur de Daidzein, est un inhibiteur compétitif de l'ATP de Cot (Tpl2/MAP3K8) et MKK4. 3',4',7-Trihydroxyisoflavone a des activités anticancéreuses, anti-angiogéniques, chimioprotectrices et antiradicalaires.
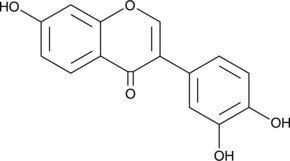
-
GC48395
3,3',5-Triiodo-L-thyronine (sodium salt hydrate)
3,3',5-Triiodo-L-thyronine (sel de sodium hydraté) est une forme active de l'hormone thyroÏdienne.
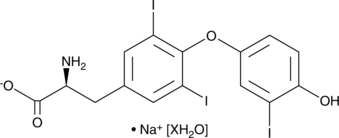
-
GC42203
3,4',5-Trismethoxybenzophenone
Resveratrol is a potent phenolic antioxidant found in natural sources that has antiproliferative activity.
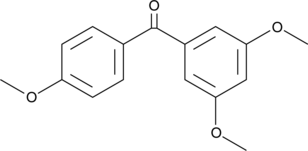
-
GC46557
3,4-Dihydroquinolin-2(1H)-one
A building block
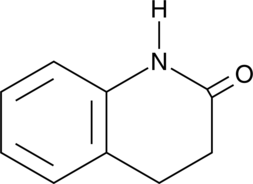
-
GC46577
3,5-Dihydroxybenzaldehyde
A building block
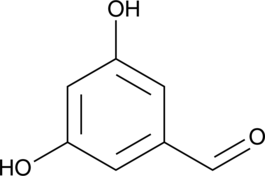
-
GC18205
3,5-Diiodothyroacetic Acid
3,5-Diiodothyroacetic acid (diac) is the acetic acid variant of thyroxine.
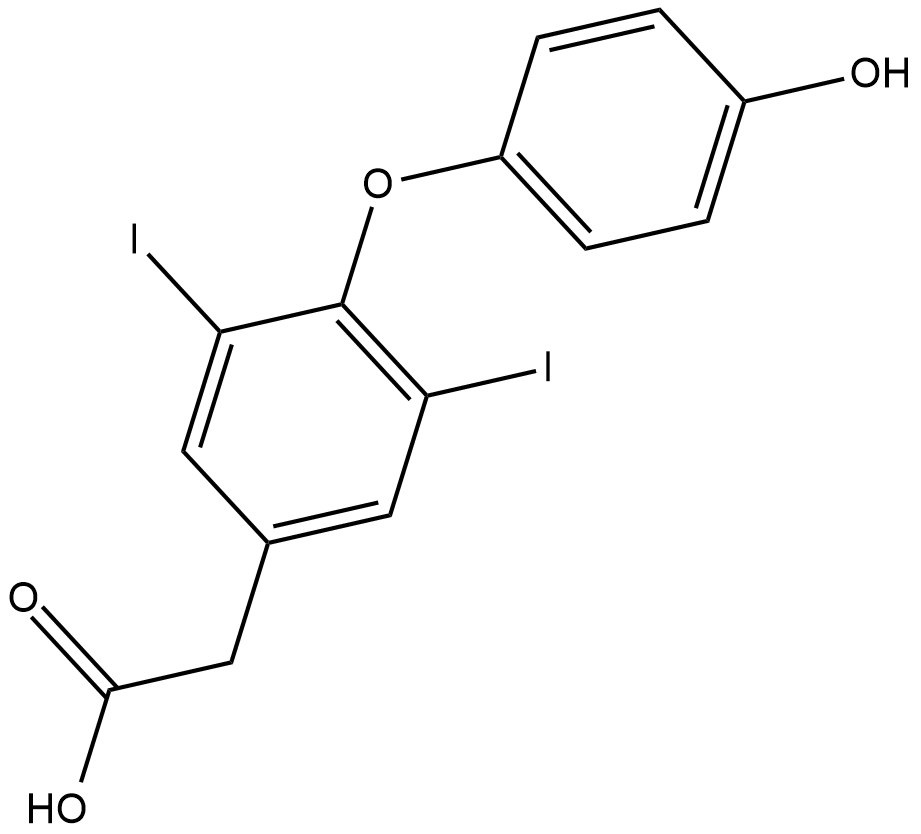
-
GC49169
3,8’-Biapigenin
3,8’-Biapigenine est une biflavone dans Hypericum perforatum L.
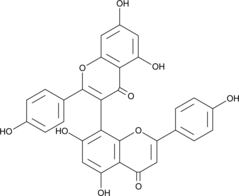
-
GC52324
3-(3-Hydroxyphenyl)propionic Acid sulfate
A metabolite of certain phenols and glycosides
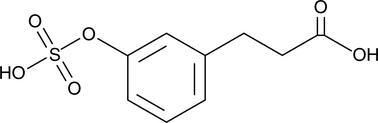
-
GC49849
3-Aminosalicylic Acid
A salicylic acid derivative
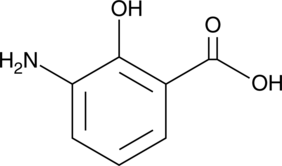
-
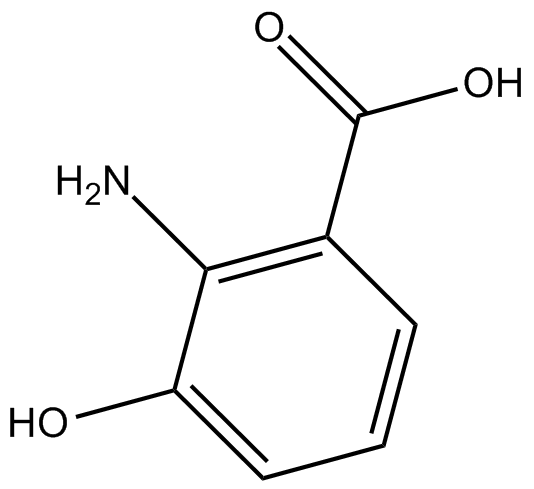
GC13377
3-hydroxy Anthranilic Acid
L'acide 3-hydroxy anthranilique est un métabolite du tryptophane dans la voie de la kynurénine.
-
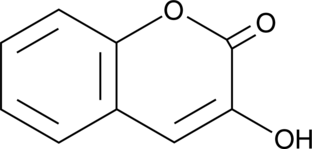
GC49364
3-Hydroxycoumarin
La 3-hydroxycoumarine est un inhibiteur puissant et redox de la 15-LOX-1 humaine.
-
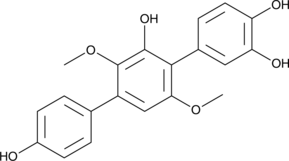
GC45337
3-Hydroxyterphenyllin
La 3-hydroxyterphénylline est un métabolite d'Aspergillus candidus. La 3-hydroxyterphénylline supprime la prolifération et provoque une cytotoxicité contre les cellules A2780/CP70 et OVCAR-3. La 3-hydroxyterphénylline induit l'arrêt de la phase S et l'apoptose. La 3-hydroxyterphénylline a le potentiel pour la recherche sur le cancer de l'ovaire.
-
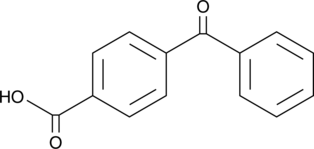
GC46609
4-(Phenylcarbonyl)benzoic Acid
A photooxidant
-
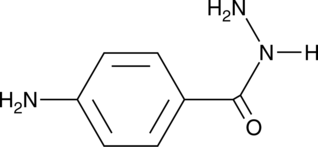
GC42338
4-Aminobenzoic Acid hydrazide
L'hydrazide d'acide 4-aminobenzoÏque est un inhibiteur irréversible de la myéloperoxydase MPO avec une IC50 de 0,3 μM.
-
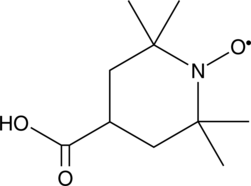
GC42351
4-carboxy TEMPO
4-carboxy TEMPO is a nitroxide and spin label.
-
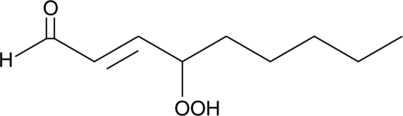
GC42400
4-hydroperoxy 2-Nonenal
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids, such as linoleic acid and arachidonic acid, that is widely used as a marker of oxidative stress.
-
GC18858
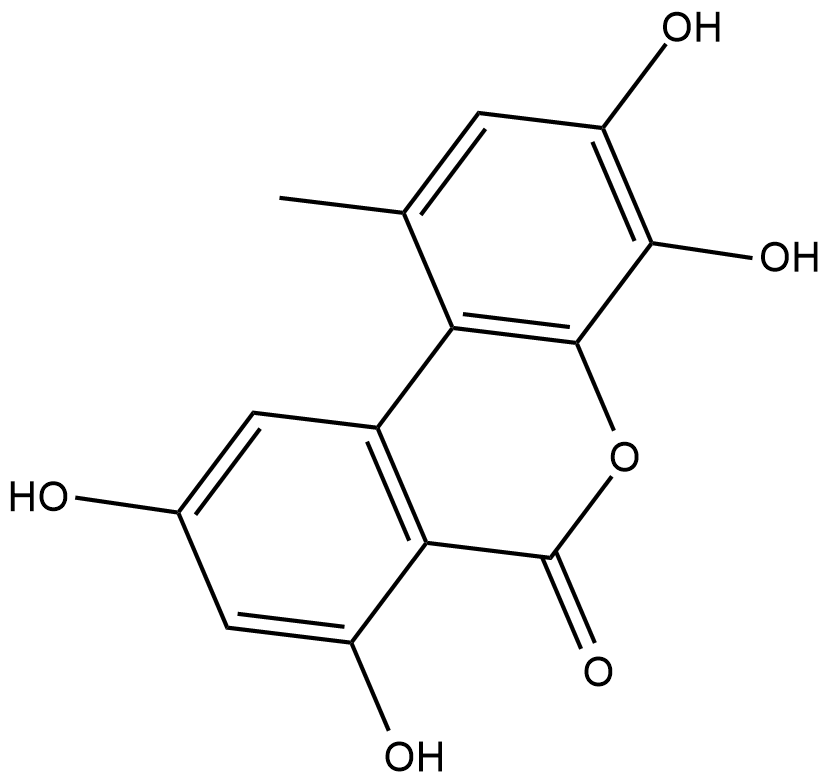
4-hydroxy Alternariol
4-hydroxy Alternariol is a metabolite of the mycotoxin alternariol formed through cytochrome P450 (CYP450) metabolism.
-
GC48824
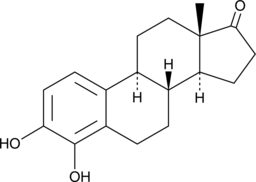
4-hydroxy Estrone
La 4-hydroxyestrone (4-OHE1), un métabolite de l'estrone, a un fort effet neuroprotecteur contre la neurotoxicité oxydative.
-
GC40778
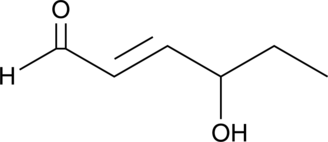
4-hydroxy Hexenal
4-hydroxy Hexenal is a lipid peroxidation product derived from oxidized ω-3 fatty acids such as DHA.
-
GC46656
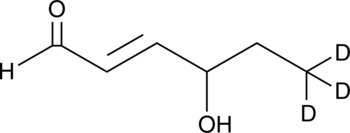
4-hydroxy Hexenal-d3
An internal standard for the quantification of 4-hydroxy hexenal
-
GC42411
4-hydroxy Nonenal Alkyne
4-hydroxy Nonenal (4-HNE) is a major aldehyde produced during the lipid peroxidation of ω-6 polyunsaturated fatty acids, such as arachidonic acid and linoleic acid.
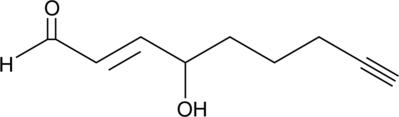
-
GC42413
4-hydroxy Nonenal Mercapturic Acid
Peroxidation of common ω-6 polyunsaturated fatty acids (PUFAs) such as linoleic acid, DGLA, and arachidonic acid can give rise to 4-HNE.
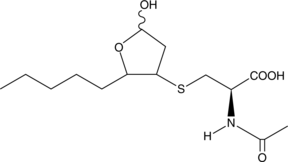
-
GA20418
4-Hydroxy-hippuric acid
Polyphenol metabolite.

-
GC42464
4-oxo-2-Nonenal
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids such as arachidonic acid and linoleic acid.
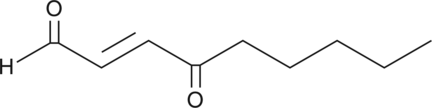
-
GC40498
4-oxo-2-Nonenal Alkyne
4-oxo-2-Nonenal is a product of lipid peroxidation that actively modifies histidine and lysine residues on proteins and causes protein cross-linking.
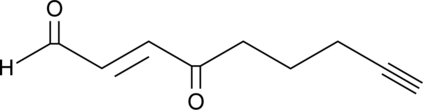
-
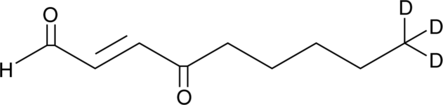
GC46674
4-oxo-2-Nonenal-d3
An internal standard for the quantification of 4oxo-2nonenal
-
GC40053
5α,6α-epoxy Cholestanol
An oxysterol and a metabolite of cholesterol produced by oxidation

-
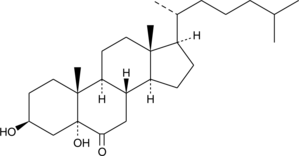
GC40693
5α-hydroxy-6-keto Cholesterol
5α-hydroxy-6-céto Le cholestérol est le principal métabolite de β-époxyde (5α,6β-époxycholestérol) lors de l'exposition directe À l'ozone de cellules épithéliales bronchiques humaines intactes cultivées (16-HBE).
-
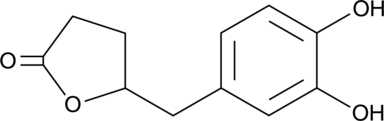
GC52227
5-(3',4'-Dihydroxyphenyl)-γ-Valerolactone
An active metabolite of various polyphenols
-
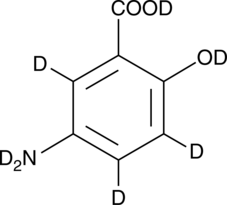
GC52413
5-Aminosalicylic Acid-d7
An internal standard for the quantification of 5-aminosalicylic acid
-
GC49233
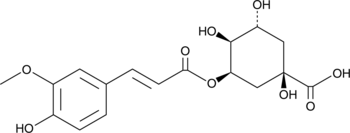
5-Feruloylquinic Acid
L'acide 5-féruloylquinique (5-FQA) possède des effets antioxydants et des activités inhibitrices de la tyrosinase.
-
GC46033
5-Heneicosylresorcinol
An alkylresorcinol

-
GC42563
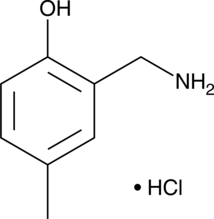
5-methyl-2-HOBA (hydrochloride)
5-methyl-2-HOBA is an isoketal scavenger.
-
GC46079
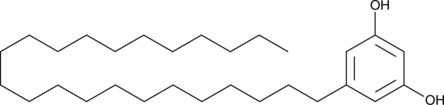
5-Tricosylresorcinol
Le 5-tricosylresorcinolthe est le premier lipide du kyste.
-
GC45772
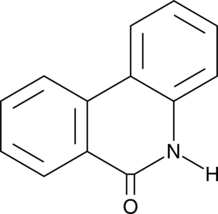
6(5H)-Phenanthridinone
An inhibitor of PARP1 and 2
-
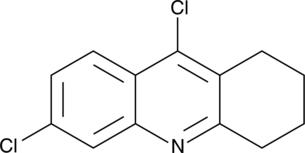
GC46720
6,9-Dichloro-1,2,3,4-tetrahydroacridine
A synthetic intermediate in the synthesis of AChE inhibitors