Ox Stress Reagents
- Antioxidants(219)
- Carbohydrate Oxidation(3)
- Free Radical Generators(4)
- Hydrogen Sulfide Donors(5)
- Lipid Peroxidation(168)
- NO Donors(17)
- Pro-Oxidant Activity(42)
- Protein Oxidation(13)
- Spin Traps(9)
Products for Ox Stress Reagents
- Cat.No. Product Name Information
-
GC11123
α-CEHC
A major metabolite of δ-tocopherol

-
GC52253
α-Enolase (1-19)-biotin Peptide
A biotinylated α-enolase peptide
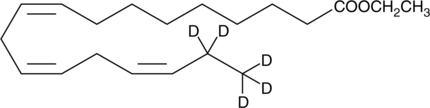
-
GC45601
α-Linolenic Acid ethyl ester-d5
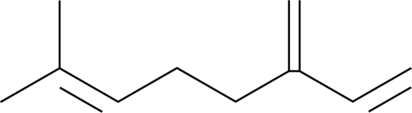
-
GC41502
β-Myrcene
β-Myrcene is a terpene that has been found in Cannabis and has antioxidative properties.
-
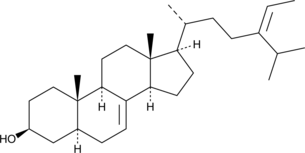
GC48317
δ7-Avenasterol
A phytosterol that has antioxidant activities
-
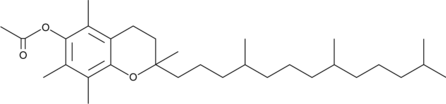
GC45713
(±)-α-Tocopherol Acetate
(±)-α-Tocopherol Acetate ((±)-Vitamin E acetate), is a orally active synthetic form of vitamin E.
-
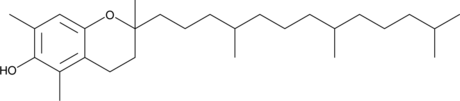
GC40015
(±)-5,7-Dimethyltocol
(±)-5,7-Dimethyltocol is a form of tocopherol.
-
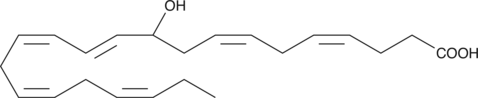
GC41213
(±)10-HDHA
(±)10-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC41214
(±)11-HDHA
(±)11-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
GC40428
(±)11-HEDE
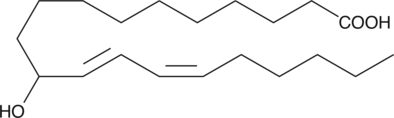
(±)11-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.
-
GC40467
(±)11-HETE
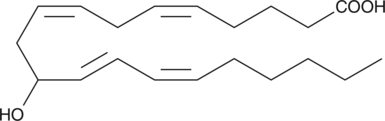
(±)11-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC40359
(±)12-HEPE
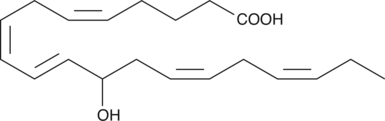
(±)12-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC40429
(±)12-HETE
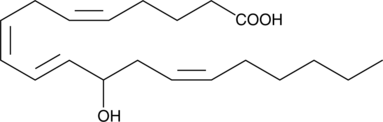
(±)12-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC41192
(±)13-HDHA
(±)13-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
GC41649
(±)13-HODE cholesteryl ester
(±)13-HODE cholesteryl ester was originally extracted from atherosclerotic lesions and shown to be produced by Cu2+-catalyzed oxidation of LDL.

-
GC40355
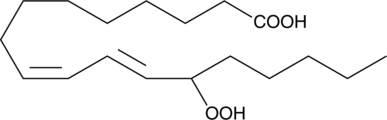
(±)13-HpODE
(±)13-HpODE is a racemic mixture of hydroperoxides derived non-enzymatically from linoleic acid through the action of reactive oxygen species.
-
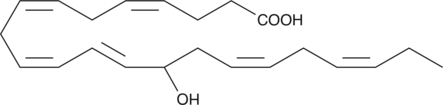
GC41193
(±)14-HDHA
(±)14-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
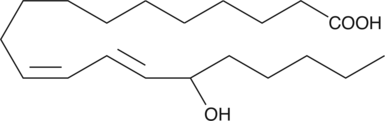
GC40420
(±)15-HEDE
(±)15-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.
-
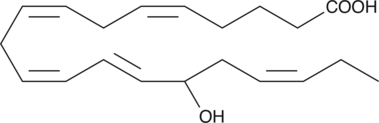
GC40361
(±)15-HEPE
(±)15-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC41196
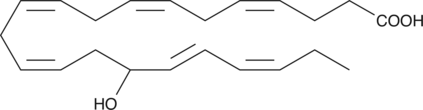
(±)16-HDHA
(±)16-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC41197
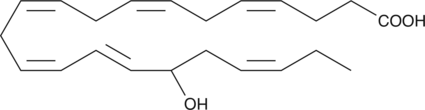
(±)17-HDHA
(±)17-HDHA is an autoxidation product of docosahexaenoic acid in vitro.
-
GC40362
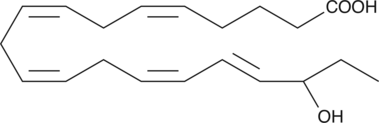
(±)18-HEPE
(±)18-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC41202
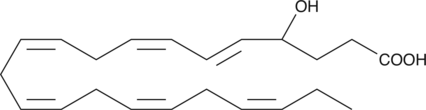
(±)4-HDHA
(±)4-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC40364
(±)5-HEPE
(±)5-HEPE is produced by non-enzymatic oxidation of EPA.
-
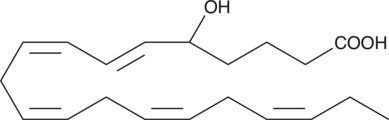
GC40439
(±)5-HETE
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
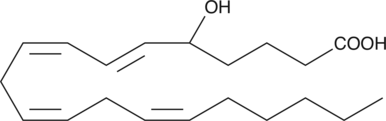
GC40828
(±)5-HETE lactone
(±)5-HETE lactone is a cyclic ester formed by acid-catalyzed nucleophilic addition of the C-5 hydroxyl to the C-1 carboxyl of (±)5-HETE.

-
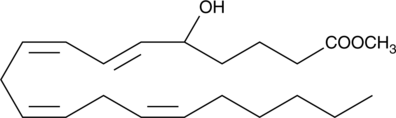
GC40837
(±)5-HETE methyl ester
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
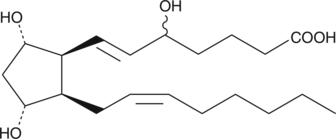
GC40576
(±)5-iPF2α-VI
Isoprostanes are prostaglandin (PG)-like products of free-radical induced lipid peroxidation.
-
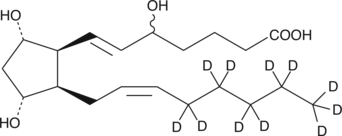
GC46263
(±)5-iPF2α-VI-d11
An internal standard for the quantification of (±)5iPF2αVI
-
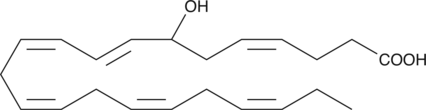
GC41204
(±)7-HDHA
(±)7-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
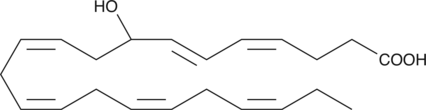
GC41205
(±)8-HDHA
(±)8-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
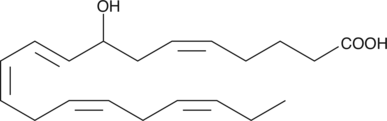
GC40366
(±)8-HEPE
(±)8-HEPE is produced by non-enzymatic oxidation of EPA.
-
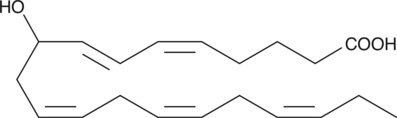
GC40367
(±)9-HEPE
(±)9-HEPE is produced by non-enzymatic oxidation of EPA.
-
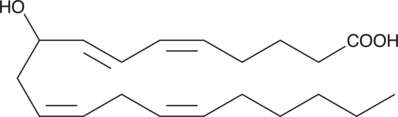
GC40443
(±)9-HETE
(±)9-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
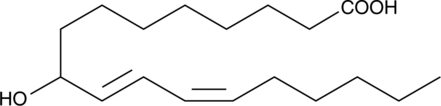
GC40541
(±)9-HODE
(±)9-HODE is one of the two racemic monohydroxy fatty acids resulting from the non-enzymatic oxidation of linoleic acid.
-
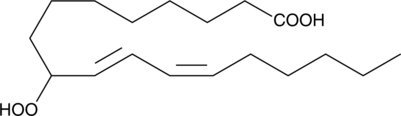
GC40356
(±)9-HpODE
(±)9-HpODE is a racemic mixture of the fatty acid hydroperoxide product (9(S)-HpODE) formed from lipoxygenase action on linoleic acid.
-
GC45248
(-)-FINO2
(-)-FINO2 is a ferroptosis-inducing peroxide compound that indirectly inhibits glutathione peroxidase 4 (GPX4) and oxidizes iron.
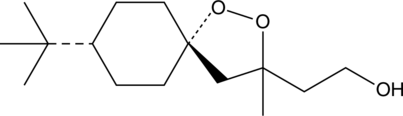
-
GC49690
(3R,5R)-Rosuvastatin (calcium salt)
A potential impurity found in bulk preparations of rosuvastatin
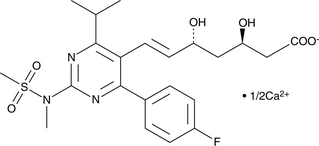
-
GC41700
(E)-2-(2-Chlorostyryl)-3,5,6-trimethylpyrazine
(E)-2-(2-Chlorostyryl)-3,5,6-trimethylpyrazine (CSTMP) is a stilbene derivative with antioxidant and anticancer activities.
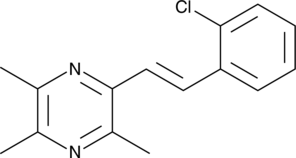
-
GC49003
(E)-Ajoene
A disulfide with diverse biological activities
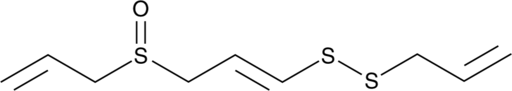
-
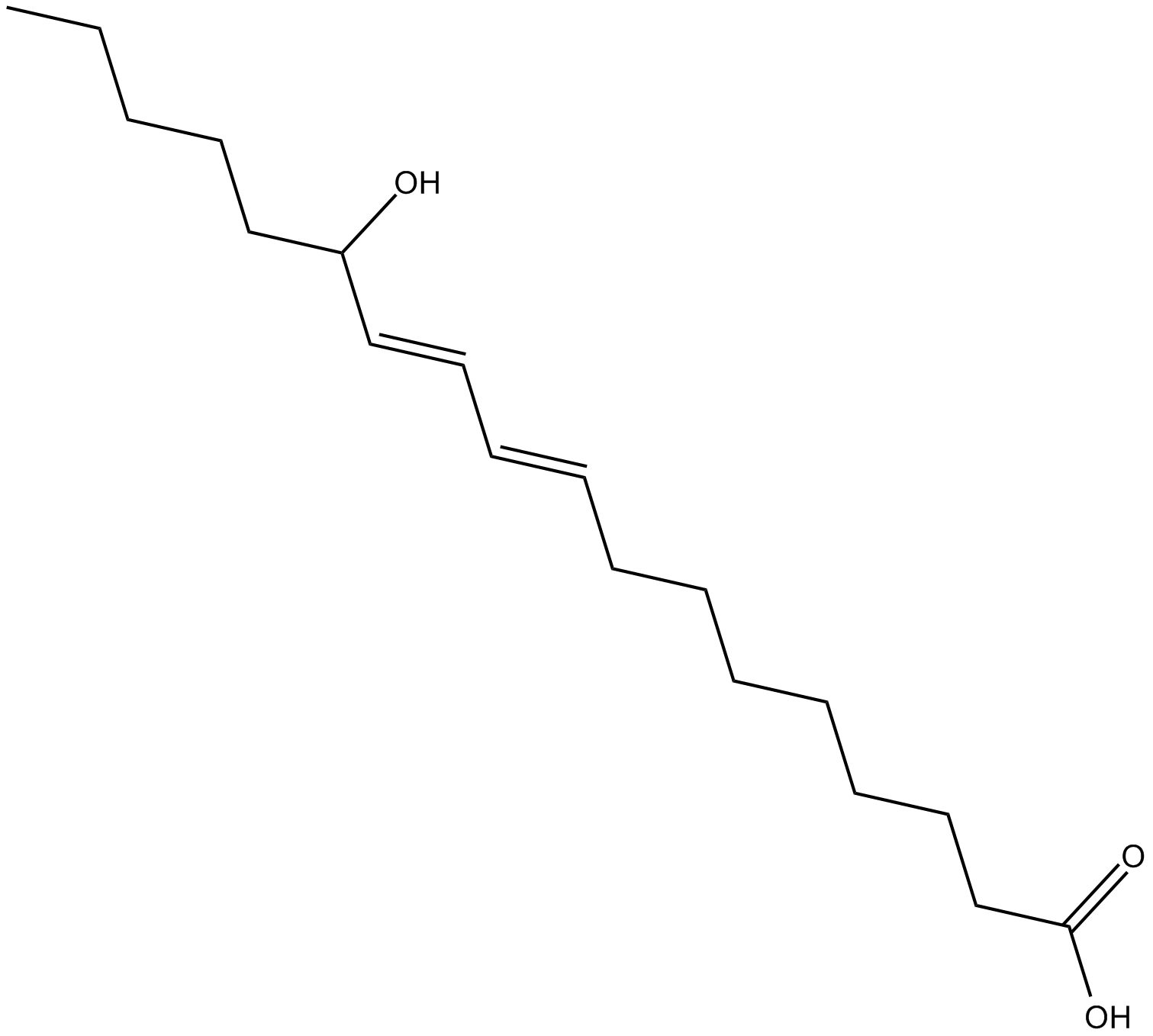
GC19461
(±)13-HODE
(±)13-HODE is one of the two racemic monohydroxy fatty acids resulting from the non-enzymatic oxidation of linoleic acid.
-
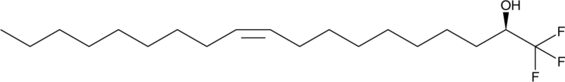
GC49034
1(R)-(Trifluoromethyl)oleyl alcohol
An oleic acid analog
-
GC41837
1,3,7-Trimethyluric Acid
1,3,7-Trimethyluric acid is a methyl derivative of uric acid and a product of C-8 oxidation of caffeine by cytochrome P450 enzymes.

-
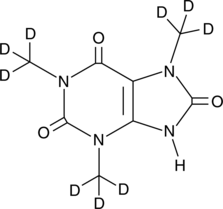
GC46387
1,3,7-Trimethyluric Acid-d9
An internal standard for the quantification of 1,3,7-trimethyluric acid
-
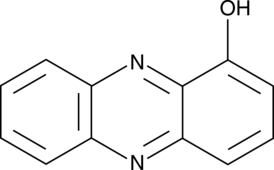
GC46481
1-Hydroxyphenazine
A P. aeruginosa virulence factor
-
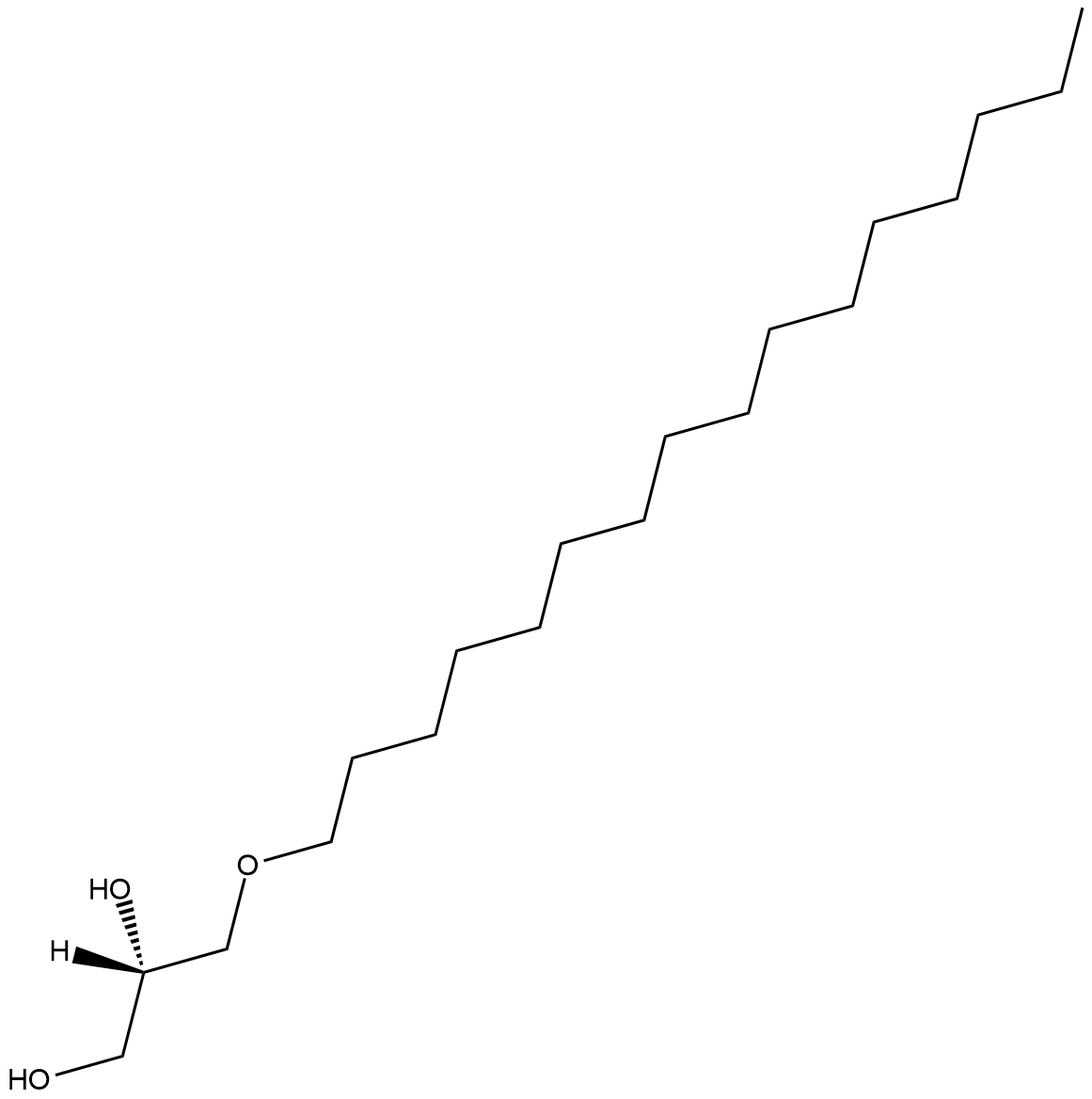
GC18235
1-O-Hexadecyl-sn-glycerol
1-O-Hexadecyl-sn-glycerol is a bioactive alkyl glyceryl ether.
-
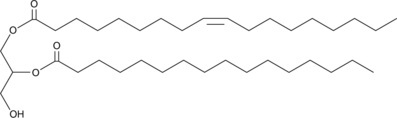
GC40146
1-Oleoyl-2-Palmitoyl-rac-glycerol
1-Oleoyl-2-palmitoyl-rac-glycerol (1,2-OP) is a diacylglycerol containing oleic acid at the sn-1 position and palmitic acid at the sn-2 position.
-
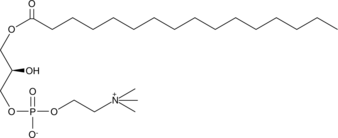
GC42026
1-Palmitoyl-2-hydroxy-sn-glycero-3-PC
1-Palmitoyl-2-hydroxy-sn-glycero-3-PC is an abundant gonadal LPC (lysophosphatidylcholine).
-
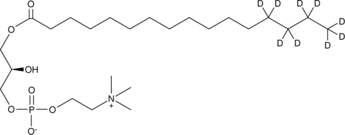
GC45693
1-Palmitoyl-d9-2-hydroxy-sn-glycero-3-PC
A quantitative analytical standard guaranteed to meet MaxSpec® identity, purity, stability, and concentration specifications
-
GC48782
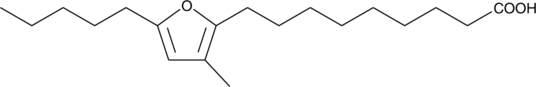
10,13-epoxy-11-methyl-Octadecadienoic Acid
A furan fatty acid
-
GC41866
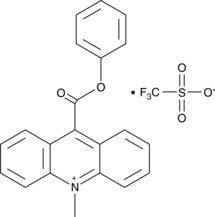
10-methyl-9-(phenoxycarbonyl) Acridinium (trifluoromethylsulfonate)
10-methyl-9-(phenoxycarbonyl) Acridinium is an acridinium ester that produces fluorescent 10-methyl-9-acridone upon oxidation with hydrogen peroxide, persulfates, and other oxidants in alkaline conditions.
-
GC41868
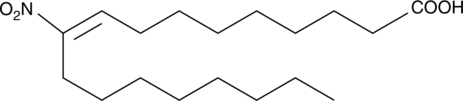
10-Nitrooleate
10-Nitrooleate (CXA-10), a nitro fatty acid, has potential effects in disease states in which oxidative stress, inflammation, fibrosis, and/or direct tissue toxicity play significant roles.
-
GC41893
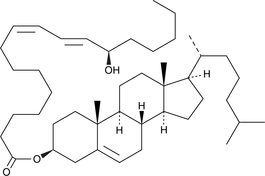
13(R)-HODE cholesteryl ester
13(R)-HODE cholesteryl ester was originally extracted from atherosclerotic lesions.
-
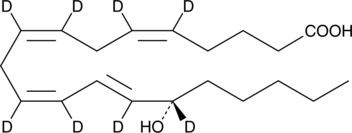
GC46442
15(S)-HETE-d8
An internal standard for the quantification of 15-HETE
-
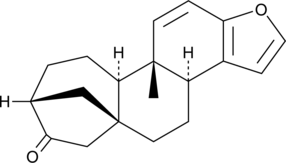
GC46452
16-Oxokahweol
A synthetic diterpene
-
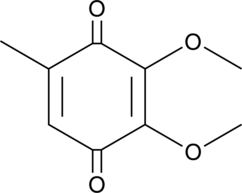
GC40947
2,3-Dimethoxy-5-methyl-p-benzoquinone
2,3-Dimethoxy-5-methyl-p-benzoquinone (CoQ0) is a potent, oral active ubiquinone compound can be derived from Antrodia cinnamomea.
-
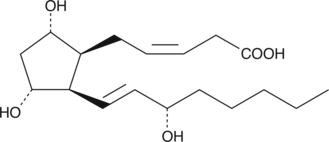
GC40416
2,3-dinor-8-iso Prostaglandin F2α
8-iso Prostaglandin F2α (8-iso PGF2α; 8-isoprostane) is a prostaglandin-like product of non-specific lipid peroxidation.
-
GC46057
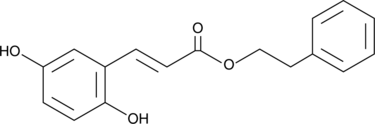
2,5-Dihydroxycinnamic Acid phenethyl ester
An inhibitor of 5-LO
-
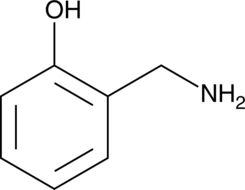
GC40503
2-HOBA
2-HOBA (2-HOBA) a selective dicarbonyl scavenger, is an antioxidant and scavanger of free radicals and isolevuglandins (IsoLGs).
-
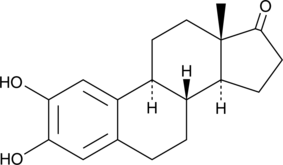
GC49172
2-hydroxy Estrone
An active metabolite of estrone
-
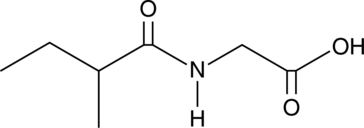
GC49840
2-Methylbutyrylglycine
A metabolite of isoleucine
-
GC42195
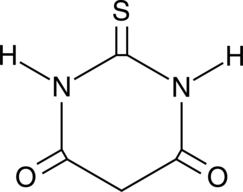
2-Thiobarbituric Acid
2-Thiobarbituric acid is a colorimetric reagent commonly used in the detection of malondialdehyde (MDA), a marker of lipid peroxidation.
-
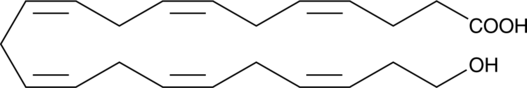
GC41210
22-HDHA
22-HDHA is an oxidation product of docosahexaenoic acid.
-
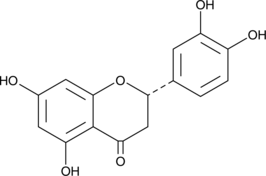
GC49005
2S-Eriodictyol
A flavanone with antioxidant activity
-
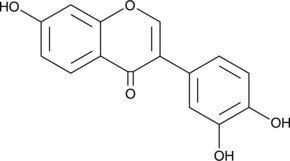
GC40618
3',4',7-Trihydroxyisoflavone
3',4',7-Trihydroxyisoflavone, a major metabolite of Daidzein, is an ATP-competitive inhibitor of Cot (Tpl2/MAP3K8) and MKK4. 3',4',7-Trihydroxyisoflavone has anticancer, anti-angiogenic, chemoprotective, and free radical scavenging activities.
-
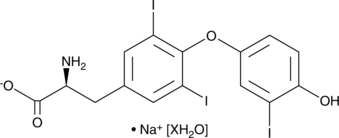
GC48395
3,3',5-Triiodo-L-thyronine (sodium salt hydrate)
3,3',5-Triiodo-L-thyronine (sodium salt hydrate) is an active form of thyroid hormone.
-
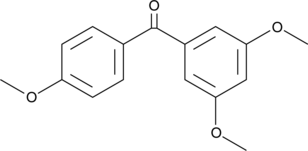
GC42203
3,4',5-Trismethoxybenzophenone
Resveratrol is a potent phenolic antioxidant found in natural sources that has antiproliferative activity.
-
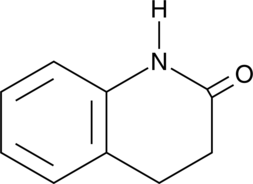
GC46557
3,4-Dihydroquinolin-2(1H)-one
A building block
-
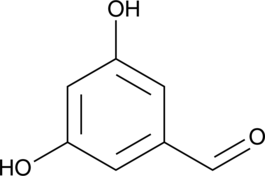
GC46577
3,5-Dihydroxybenzaldehyde
A building block
-
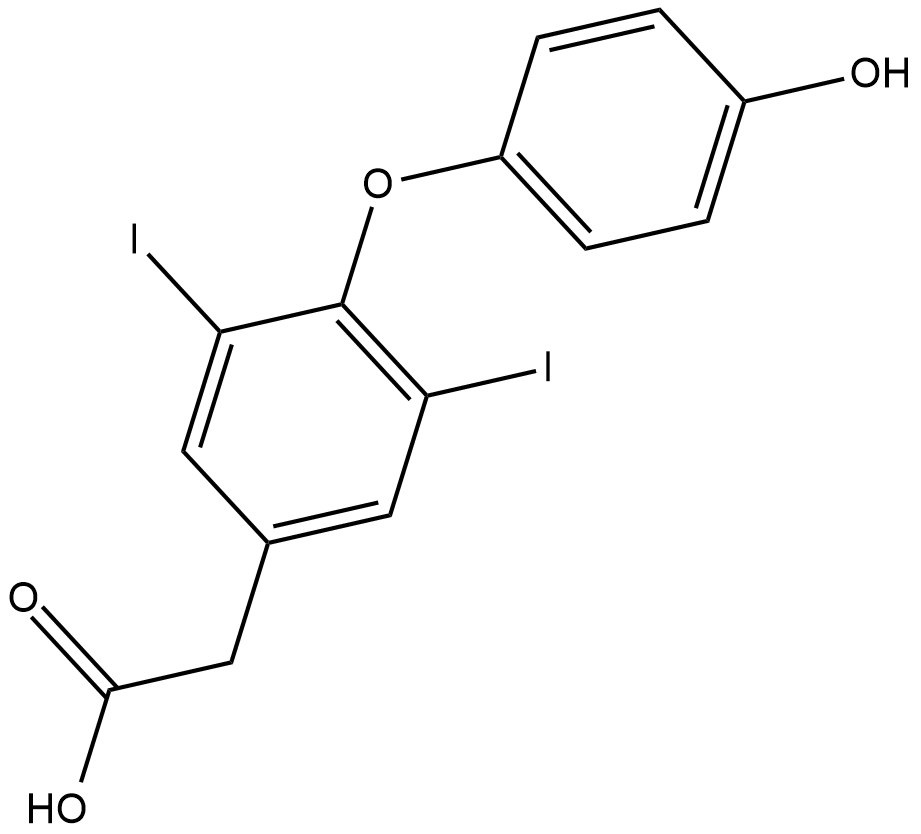
GC18205
3,5-Diiodothyroacetic Acid
3,5-Diiodothyroacetic acid (diac) is the acetic acid variant of thyroxine.
-
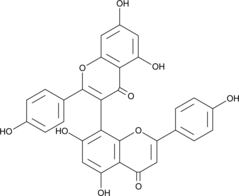
GC49169
3,8’-Biapigenin
A biflavonoid with diverse biological activities
-
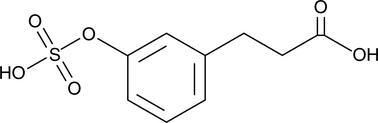
GC52324
3-(3-Hydroxyphenyl)propionic Acid sulfate
A metabolite of certain phenols and glycosides
-
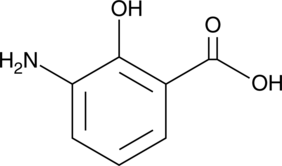
GC49849
3-Aminosalicylic Acid
A salicylic acid derivative
-
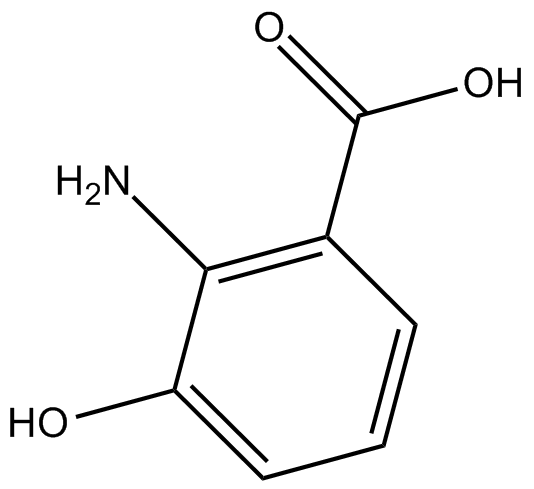
GC13377
3-hydroxy Anthranilic Acid
co-antioxidant
-
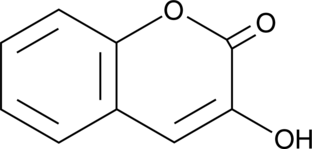
GC49364
3-Hydroxycoumarin
A coumarin with diverse biological activities
-
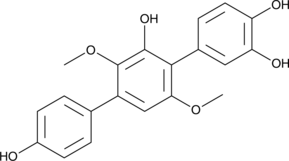
GC45337
3-Hydroxyterphenyllin
3-Hydroxyterphenyllin is a metabolite of Aspergillus candidus.3-Hydroxyterphenyllin suppresses proliferation and causes cytotoxicity against A2780/CP70 and OVCAR-3 cells. 3-Hydroxyterphenyllin induces S phase arrest and apoptosis. 3-Hydroxyterphenyllin has the potential for the research of ovarian cancer.
-
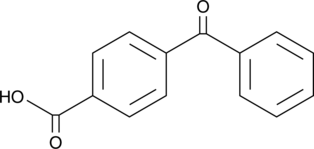
GC46609
4-(Phenylcarbonyl)benzoic Acid
A photooxidant
-
GC42338
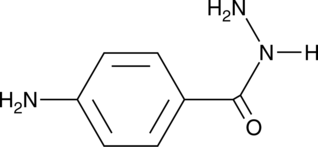
4-Aminobenzoic Acid hydrazide
4-Aminobenzoic Acid hydrazide is an irreversible MPO myeloperoxidase inhibitor with an IC50 of 0.3 μM.
-
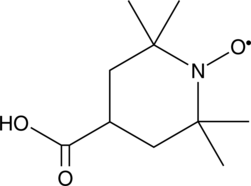
GC42351
4-carboxy TEMPO
4-carboxy TEMPO is a nitroxide and spin label.
-
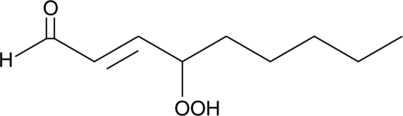
GC42400
4-hydroperoxy 2-Nonenal
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids, such as linoleic acid and arachidonic acid, that is widely used as a marker of oxidative stress.
-
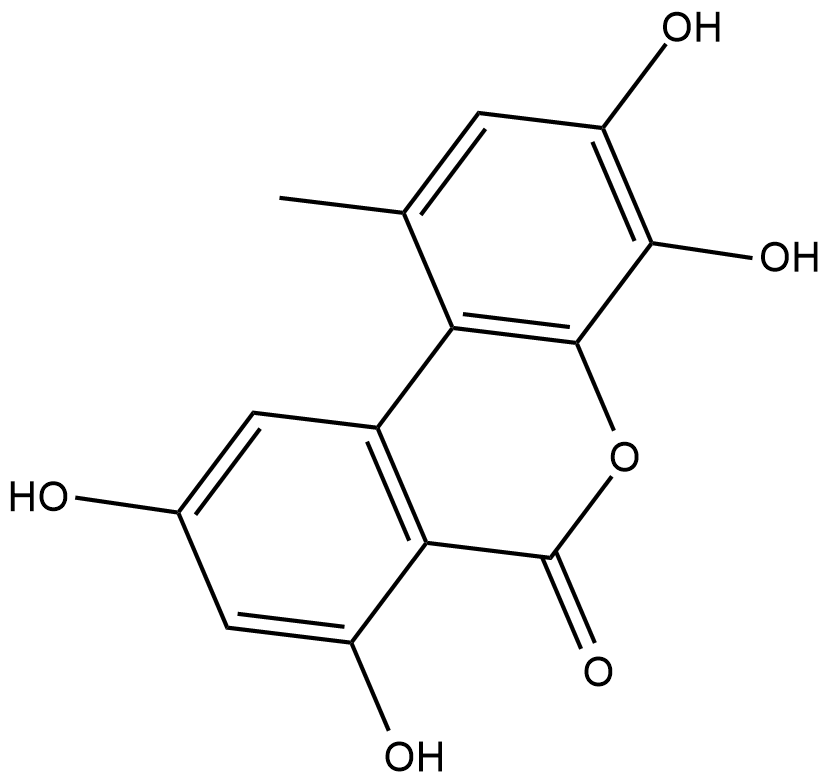
GC18858
4-hydroxy Alternariol
4-hydroxy Alternariol is a metabolite of the mycotoxin alternariol formed through cytochrome P450 (CYP450) metabolism.
-
GC48824
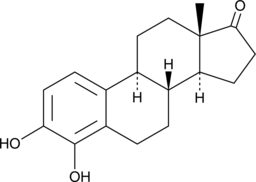
4-hydroxy Estrone
A metabolite of estrone
-
GC40778
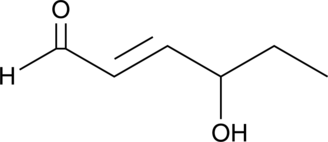
4-hydroxy Hexenal
4-hydroxy Hexenal is a lipid peroxidation product derived from oxidized ω-3 fatty acids such as DHA.
-
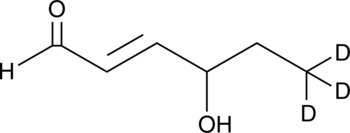
GC46656
4-hydroxy Hexenal-d3
An internal standard for the quantification of 4-hydroxy hexenal
-
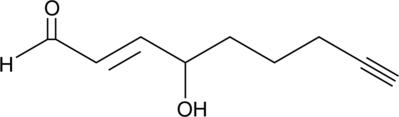
GC42411
4-hydroxy Nonenal Alkyne
4-hydroxy Nonenal (4-HNE) is a major aldehyde produced during the lipid peroxidation of ω-6 polyunsaturated fatty acids, such as arachidonic acid and linoleic acid.
-
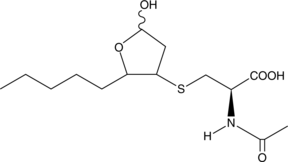
GC42413
4-hydroxy Nonenal Mercapturic Acid
Peroxidation of common ω-6 polyunsaturated fatty acids (PUFAs) such as linoleic acid, DGLA, and arachidonic acid can give rise to 4-HNE.
-
GA20418
4-Hydroxy-hippuric acid
Polyphenol metabolite.

-
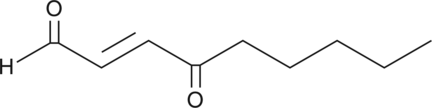
GC42464
4-oxo-2-Nonenal
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids such as arachidonic acid and linoleic acid.
-
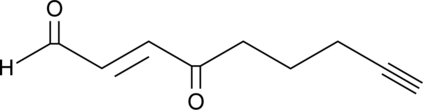
GC40498
4-oxo-2-Nonenal Alkyne
4-oxo-2-Nonenal is a product of lipid peroxidation that actively modifies histidine and lysine residues on proteins and causes protein cross-linking.
-
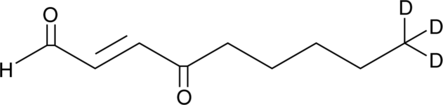
GC46674
4-oxo-2-Nonenal-d3
An internal standard for the quantification of 4oxo-2nonenal
-
GC40053
5α,6α-epoxy Cholestanol
An oxysterol and a metabolite of cholesterol produced by oxidation

-
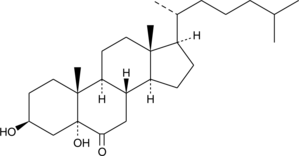
GC40693
5α-hydroxy-6-keto Cholesterol
Cholesterol is the most abundant neutral lipid present in the surfactant of the lung epithelial lining fluid.
-
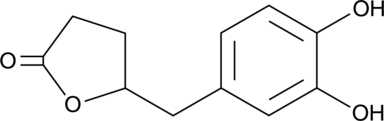
GC52227
5-(3',4'-Dihydroxyphenyl)-γ-Valerolactone
An active metabolite of various polyphenols
-
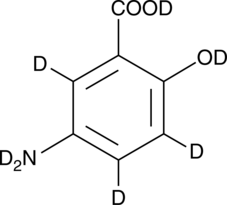
GC52413
5-Aminosalicylic Acid-d7
An internal standard for the quantification of 5-aminosalicylic acid
-
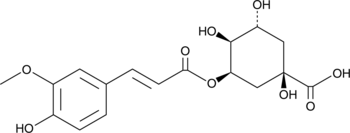
GC49233
5-Feruloylquinic Acid
A chlorogenic acid with antioxidant activity
-
GC46033
5-Heneicosylresorcinol
An alkylresorcinol

-
GC42563
5-methyl-2-HOBA (hydrochloride)
5-methyl-2-HOBA is an isoketal scavenger.
-
GC46079
5-Tricosylresorcinol
5-Tricosylresorcinolthe is the first cyst lipid.
-
GC45772
6(5H)-Phenanthridinone
An inhibitor of PARP1 and 2
-
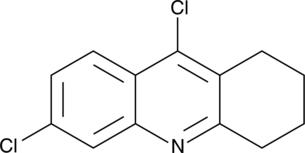
GC46720
6,9-Dichloro-1,2,3,4-tetrahydroacridine
A synthetic intermediate in the synthesis of AChE inhibitors