Arecoline hydrobromide |
| カタログ番号GC10264 |
ムスカリン性アセチルコリン受容体作動薬
Products are for research use only. Not for human use. We do not sell to patients.
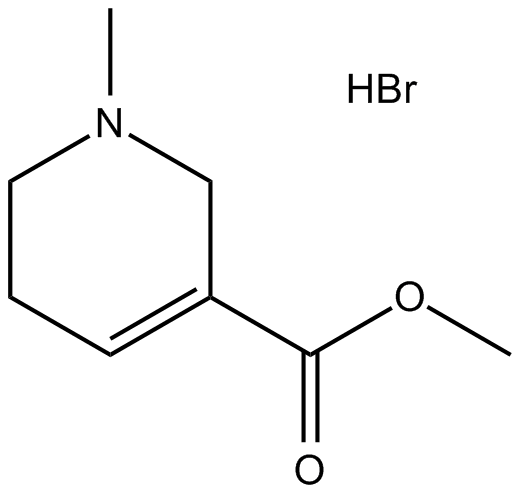
Cas No.: 300-08-3
Sample solution is provided at 25 µL, 10mM.
Arecoline hydrobromide is a nicotinic acids-based alkaloid and partial agonist of muscarinic acetylchiline M1, M2, M3 and M4 receptors. The LD50 of subcutaneous injection value is 100 mg/kg in mouse [1].
Arecoline hydrobromide has been suggested to have electrophysiological effects on ventricular myocytes. Arecoline hydrobromide has shown the significant inhibitory effect on hERG current with the IC50 value of 9.55μmol/L. Furthermore, Arecoline hydrobromide has demonstrated the concentration- , time- and voltage-dependent characteristics of IhERG inhibition in the patch clamp experiments. Apart from these, Arecoline hydrobromide has been exhibited to significant change in the amount of steady-state blockade at stimulation frequencies. It has been shown frequency-dependent characteristics inhibition of IhERG by Arecoline hydrobromide [1].
References:
[1] Zhao XY1, Liu YQ, Fu YC, Xu B, Gao JL, Zheng XQ, Lin M, Chen MY, Li Y. Frequency- and state-dependent blockade of human ether-a-go-go-related gene K+ channel by arecoline hydrobromide. Chin Med J (Engl). 2012 Mar;125(6):1068-75.
Average Rating: 5 (Based on Reviews and 16 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















