Pamiparib (BGB-290) (Synonyms: BGB-290) |
| Catalog No.GC34071 |
파미파립(BGB-290)(BGB-290)은 경구 활성, 강력하고 고도로 선택적인 PARP 억제제이며 IC50 값은 PARP1 및 PARP2에 대해 각각 0.9nM 및 0.5nM입니다. 파미파립(BGB-290)은 강력한 PARP 포집 능력과 뇌 침투 능력을 갖고 있어 고형암을 비롯한 다양한 암 연구에 활용될 수 있다.
Products are for research use only. Not for human use. We do not sell to patients.
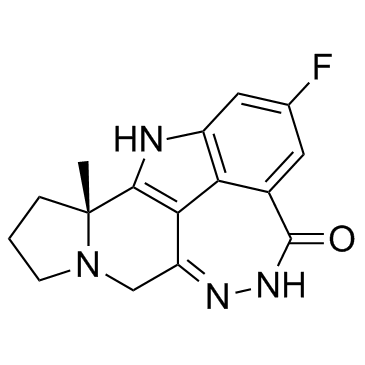
Cas No.: 1446261-44-4
Sample solution is provided at 25 µL, 10mM.
Pamiparib (BGB-290) is an orally active, potent, highly selective PARP inhibitor, with IC50 values of 0.9 nM and 0.5 nM for PARP1 and PARP2, respectively. Pamiparib has oral bioavailability, potent PARP trapping, and capability to penetrate the brain, and can be used for the treatment of various cancers including the solid tumor[1][2].
Pamiparib shows potent DNA-trapping activity with an IC50 of 13 nM. In the cellular assays, Pamiparib inhibits intracellular PAR formation with an IC50 of 0.24 nM. Tumor cell lines with homologous recombination defects are profoundly sensitive to Pamiparib. Pamiparib is highly active both in vitro and in vivo in BRCA mutant tumors[3].
Pamiparib suppresses PARP activity in patient-derived glioblastoma multiforme and small-cell-lung cancer xenografts, and potentiates the effects of Temozolamide. In vivo activities of Pamiparib, and its combination activity with chemotherapies in patient biopsy derived small cell lung cancer (SCLC) xenograft models[4].
References:
[1]. Changyou Zhou, et al. Fused tetra or penta-cyclic dihydrodiazepinocarbazolones as parp inhibitors. WO 2013097225 A1.
[2]. Friedlander M, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019 Sep;20(9):1306-1315.
[3]. Zhiyu Tang, et al. Abstract 1653: BGB-290: A highly potent and specific PARP1/2 inhibitor potentiates anti-tumor activity of chemotherapeutics in patient biopsy derived SCLC models. Cancer Research. August 2015, Volume 75, Issue 15.
[4]. Shiv K. Gupta, et al. Abstract 3505: Inhibition of PARP activity by BGB-290 potentiates efficacy of NSC 362856 in patient derived xenografts of glioblastoma multiforme. Cancer Research. August 2015, Volume 75, Issue 15
Average Rating: 5 (Based on Reviews and 21 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















