Pexidartinib (PLX3397) (Synonyms: PLX3397) |
| Catalog No.GC12222 |
펙시다르티닙(PLX3397) (PLX-3397)은 강력하고 경구 투여 가능한 선택적인 ATP 경쟁적 콜로니 자극 인자 1 수용체(CSF1R 또는 M-CSFR) 및 c-Kit 억제제이며, 각각 20nM과 10nM의 IC50를 가지고 있습니다. 펙시다르티닙(PLX3397) (PLX-3397)은 다른 관련 키나아제에 비해 c-Kit 및 CSF1R에 대해 10~100배 이상의 선택성을 나타냅니다. 또한, 펙시다르티닙(PLX3397)(PLX-3397)은 세포 아포토시스를 유도하며 항암 활성을 보입니다.
Products are for research use only. Not for human use. We do not sell to patients.
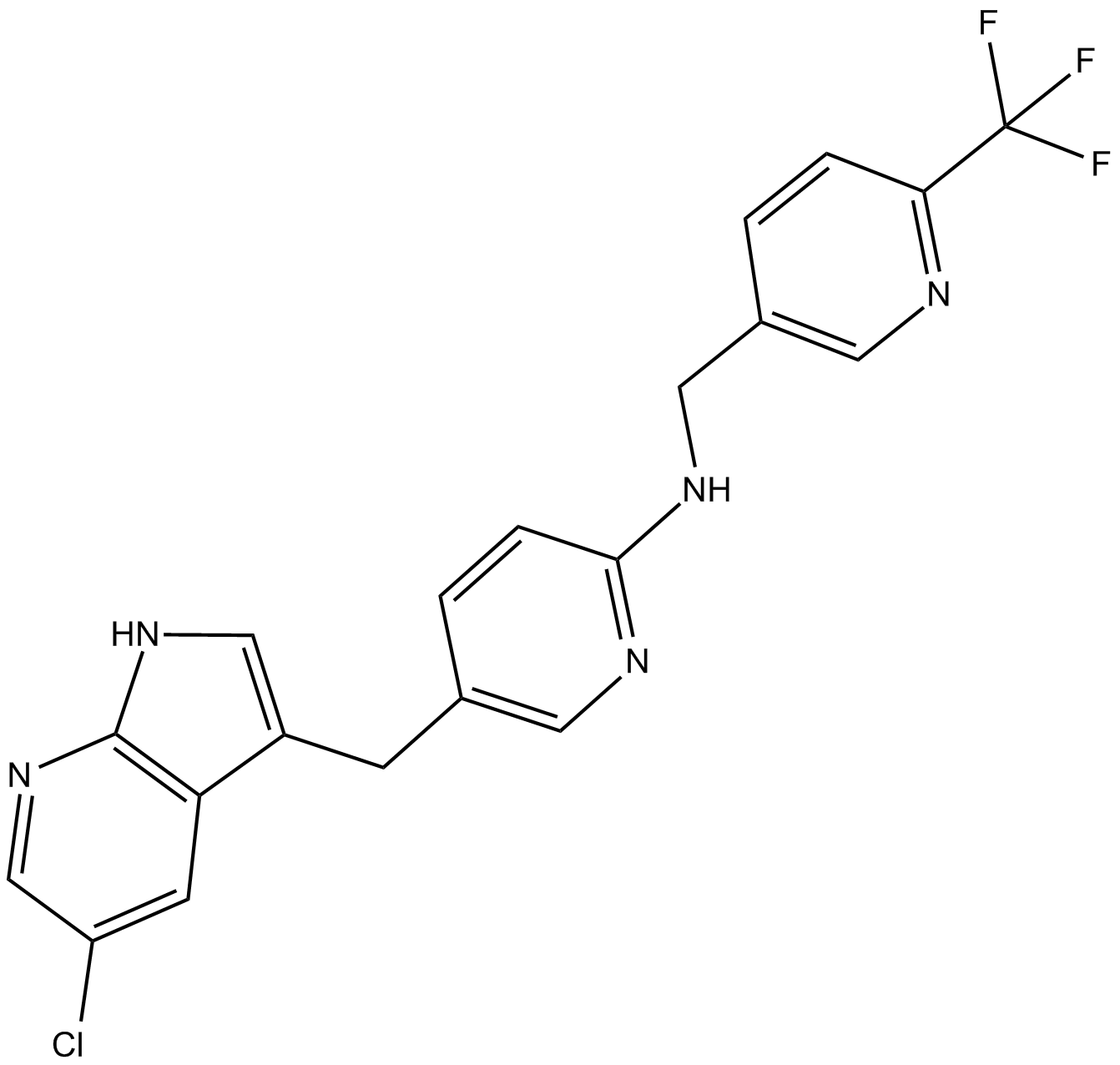
Cas No.: 1029044-16-3
Sample solution is provided at 25 µL, 10mM.
Pexidartinib(PLX3397) is an orally administered small molecule tyrosine kinase inhibitor with potent selective activity against the colony-stimulating factor 1(CSF1) receptor(IC50=20nM), KIT proto-oncogene receptor tyrosine kinase(KIT)(IC50 =10nM) and FMS-like tyrosine kinase 3[1,2]
Pexidartinib was a stronger KIT inhibitor than imatinib in vitro. Compared pexidartinib and imatinib in vitro against 2 human GIST cell lines that harbor an imatinib-sensitive, activating KIT exon 11 mutation. Indeed, pexidartinib decreased viability in both cell lines with two-fold greater potency than imatinib, with an IC50 of 8-18 nM versus 42 nM(p<0.05). At concentrations similar to the IC50 of each drug, i.e., 10 and 40 nM, PLX3397 also decreased phospho-KIT relative to total KIT more effectively than imatinib in vitro[3]
Pexidartinib is effective in reducing adipose tissue macrophage levels of chow and high fat diet mice without affecting total myeloid cell levels[4]. A research found pexidartinib was well-tolerated in non-human primates(NHPs), with no Grade 3 or Grade 4 toxicities. Pexidartinib has limited CSF penetrance in NHPs following oral administration of a single dose[5]
Pexidartinib received its first approval on 2 August 2019 in the USA for the treatment of adult patients with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery[2]
References:
[1].Fujiwara T, Yakoub MA, Chandler A, et al. CSF1/CSF1R Signaling Inhibitor Pexidartinib (PLX3397) Reprograms Tumor-Associated Macrophages and Stimulates T-cell Infiltration in the Sarcoma Microenvironment. Mol Cancer Ther. 2021;20(8):1388-1399.
[2].Lamb YN. Pexidartinib: First Approval [published correction appears in Drugs. 2020 Mar;80(4):447]. Drugs. 2019;79(16):1805-1812.
[3].Liu Y, Given KS, Dickson EL, et al. Concentration-dependent effects of CSF1R inhibitors on oligodendrocyte progenitor cells ex vivo and in vivo. Exp Neurol. 2019;318:32-41.
[4].Merry TL, Brooks AES, Masson SW, et al. The CSF1 receptor inhibitor pexidartinib (PLX3397) reduces tissue macrophage levels without affecting glucose homeostasis in mice. Int J Obes (Lond). 2020;44(1):245-253.
[5].Shankarappa PS, Peer CJ, Odabas A, et al. Cerebrospinal fluid penetration of the colony-stimulating factor-1 receptor (CSF-1R) inhibitor, pexidartinib. Cancer Chemother Pharmacol. 2020;85(5):1003-1007.
Average Rating: 5 (Based on Reviews and 25 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















