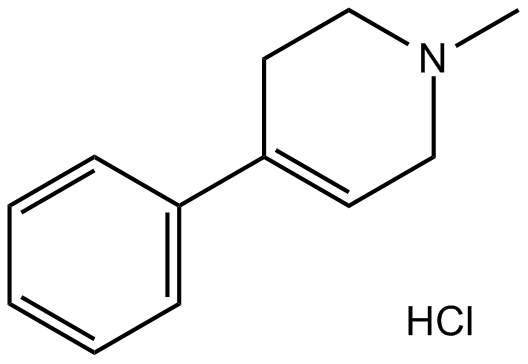
MPTP Hydrochloride
In the annals of medical research, serendipity often plays a pivotal role in leading scientists down unforeseen paths that dramatically alter our understanding of human health and disease. Such is the role of MPTP (1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine) hydrochloride, a compound whose story intertwined with the fates of a few, yet has illuminated the path for many in the battle against Parkinson’s disease. This blog article embarks on a journey through the discovery of MPTP hydrochloride, its profound impacts on neuroscience, and the ongoing quest to conquer neurodegenerative disorders.
Molecular Formula: C12H16CIN
Molecular Weight: 209.72g/mol
MPTP hydrochloride is a brain-penetrant dopamine neurotoxin. It can be used to induce Parkinson’s Disease model. MPTP hydrochloride, a precursor of MPP+, induces apoptosis. MPTP hydrochloride is used to study the mechanisms of neurotoxicity, particularly how certain substances can lead to the degeneration of neurons. Understanding MPTP's mechanism of action helps scientists gain insights into the broader principles of neuronal vulnerability and resilience, which can apply to various neurodegenerative conditions.
To confirm the importance of MPTP-induced autophagy in regulating neuronal cells by mouse primary cortical neurons were treated with 500 μM MPTP for 24 h followed by staining with Golgi-Cox or TUBB3 (tubulin, beta 3 class III) to visualize neurons. MPTP treatment reduced the length of Golgi-Cox or TUBB3-positive axons and dendrites compared to untreated neurons, and this effect could be reversed by melatonin pretreatment (Fig. 1A). We next explored the impact of MPTP treatment on the structure of neurons by evaluating the change of total dendritic length and dendritic complexity, which was demonstrated by using immunostaining for the dendritic MAP2 (microtubule-associated protein 2) (Fig. 1B). We found that both total dendritic length (Fig. 1C) and dendritic complexity (Fig. 1D) were significantly reduced in MPTP treated neurons relative to untreated neurons. Pretreatment with melatonin 14 reversed the neuronal morphological changes triggered by MPTP (Fig. 1A to D). These results indicated an active role of melatonin in regulating the neuro-morphological changes and counteracting the deleterious effect of MPTP, which would eventually prevent the dysfunction of the neurons. Similarly, MPTP treatment significantly elevated the protein level of CDK5 in a dose-dependent manner in primary cortical neurons (1E and 1F), accompanied by an increased protein level of LC3B-II (Fig. 1E and 1G) and a decreased level of SQSTM1 (Fig. 1E and 1H). Pretreatment with melatonin could restore the altered protein level of CDK5 (Fig. 1I and 1J), LC3B-II (Fig. 1I and 1K), and SQSTM1 (Fig. 1I and 1L) to the normal level.
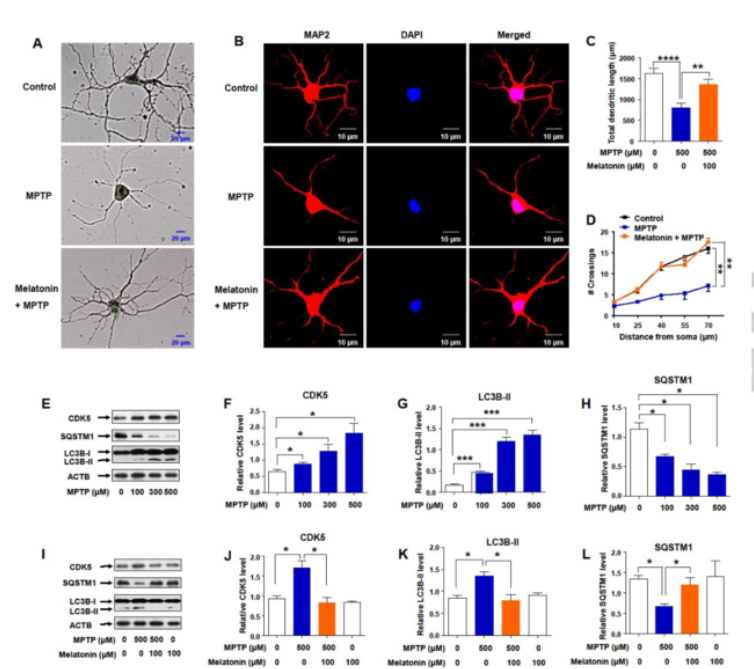
Figure 1: Effect of MPTP hydrochloride on primary neuron dendritic morphogenesis and autophagy
MPTP hydrochloride can be used in animal modeling to build Parkinson's syndrome models. MPTP reproduces naturally occurring neurodegeneration and is useful for studying dopaminergic neuron neurodegeneration, mitochondrial dysfunction, and neuroinflammation. During in vivo analysis, MPTP Hcl is dissolved in normal saline and configured when used. After administration, we can observe whether the mice have symptoms such as reduced activity, staggering walking, twitching, fried hair, increased urination, etc. This behavior may last for 24-48 hours, after which the mice behave normally.
However, there is consensus that the change in DA signaling in the nigrostriatal region is the most common outcome in PD models. Therefore, to confirm successful MPTP/p lesioning, we evaluate DA loss using immunohistochemistry for TH expression in both the substantia nigra and striatum at different time points (n = 4 mice/group at 1, 2, 8, and 16 days after acute MPTP/p treatment). The semi-quantitative results revealed that TH intensities (relative OD) in both the substantia nigra (Fig. 2A) and striatum (Fig. 2B) in the MPTP/p-lesioned group were significantly lower than those in the CON group at all time points (all P << 0.0001). Interestingly, the TH immunoreactivity in the striatum of MPTP/p-lesioned brains was substantially increased; the intensity on day 16 was approximately 44.49%, 44.24%, and 28.01% higher on days 1, 2, and 8, respectively (all P << 0.05). However, there was still a statistically significant difference in TH intensity between CON and MPTP/p-lesioned groups at each time point.
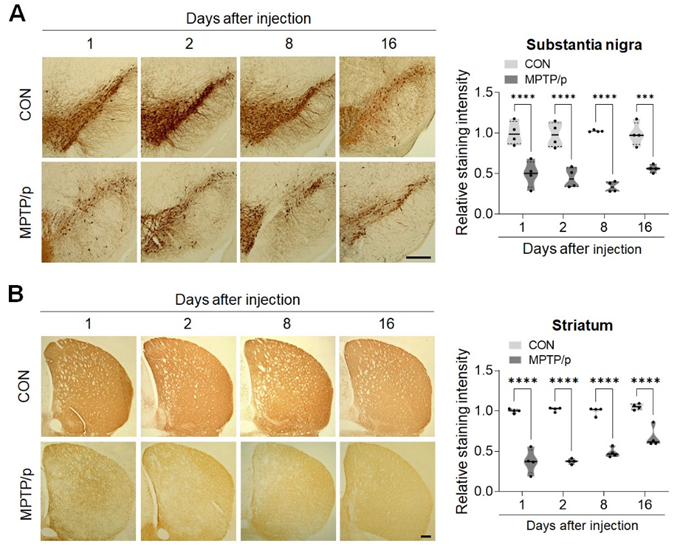
Figure 2: Acute MPTP/p treatment persistently decrease TH immunoreactivity in the nigrostriatal dopaminergic pathway.
(Left panels in A and B) Representative photomicrographs of TH protein expression in the substantia nigra (A) and striatum (B). Scale bars represent 200μm.(Violin plots in A and B) Relative ODs of TH expression were examined in the substantia nigra and striatum at different time points (1, 2, 8, and 16 days) following acute MPTP/p treatment (n = 4 mice/group). Upper and lower dashed lines signify the upper and lower quartiles, respectively, and the median is represented by a solid black line within a violin plot. ****P << 0.0001 vs. CON. CON, control (vehicle-treated group); MPTP/p, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine + probenecid-treated group; OD, optical density; TH, tyrosine hydroxylase.The MPTP hydrochloride induced model of Parkinson's disease allows for the testing of neuroprotective and neurorestorative therapies. Compounds that show promise in preventing, alleviating, or reversing the effects of MPTP toxicity may offer potential treatments for Parkinson's disease and similar disorders.
MPTP hydrochloride is a relatively simple compound that significantly impacts the understanding and treatment of PD over the past 30 years. It is a byproduct of a synthetic heroin 1-Methyl-4-phenyl-4-propionoxypiperidine. In 1982, seven young individuals acquired severe PD after they used MPTP themselves. Ballard et al. found MPTP hydrochloride in synthetic heroin causes selective destruction of dopaminergic neurons of the nigrostriatal pathway to produce PD symptoms in humans and other primates MPTP is a lipophilic compound; it can pass the blood-brain barrier into the brain and be rapidly converted to the toxic metabolite, 1-Methyl-4-phenylpyridinium or MPP+ by monoamine oxidase-B. Then MPP+ is selectively taken up by dopaminergic neurons through dopamine transporters and inhibits complex I of the mitochondrial electron transport chain, causing Parkinsonism. MPP+ severely prompts mitochondrial respiratory defects; it blocks ATP synthesis and activates free radical formation in the mitochondria of dopaminergic neurons. The MPTP-induced animal model and human PD showed similar conditions in the pathogenesis (Figure 3).
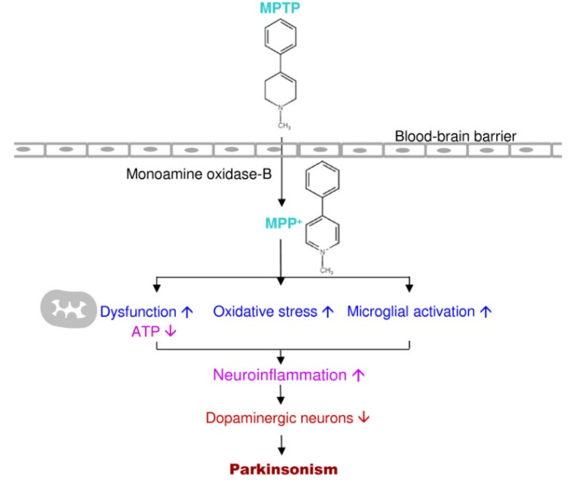
Figure 3: Mechanisms by which MPTP acts on the dopaminergic neurons in Parkinson's disease as well as its neurotoxic pathology.
The journey of MPTP hydrochloride from an accidental neurotoxin to a linchpin in Parkinson's disease research is a testament to the unpredictable nature of scientific exploration. It underscores the resilience of the human spirit and the capacity for tragedy to foster innovation and hope. As we forge ahead in our understanding of neurodegenerative diseases, MPTP hydrochloride stands as a beacon, reminding us of the serendipitous discoveries that can emerge from the most unexpected circumstances and the enduring quest for knowledge that drives us forward.












Kommentare