(-)-p-Bromotetramisole Oxalate
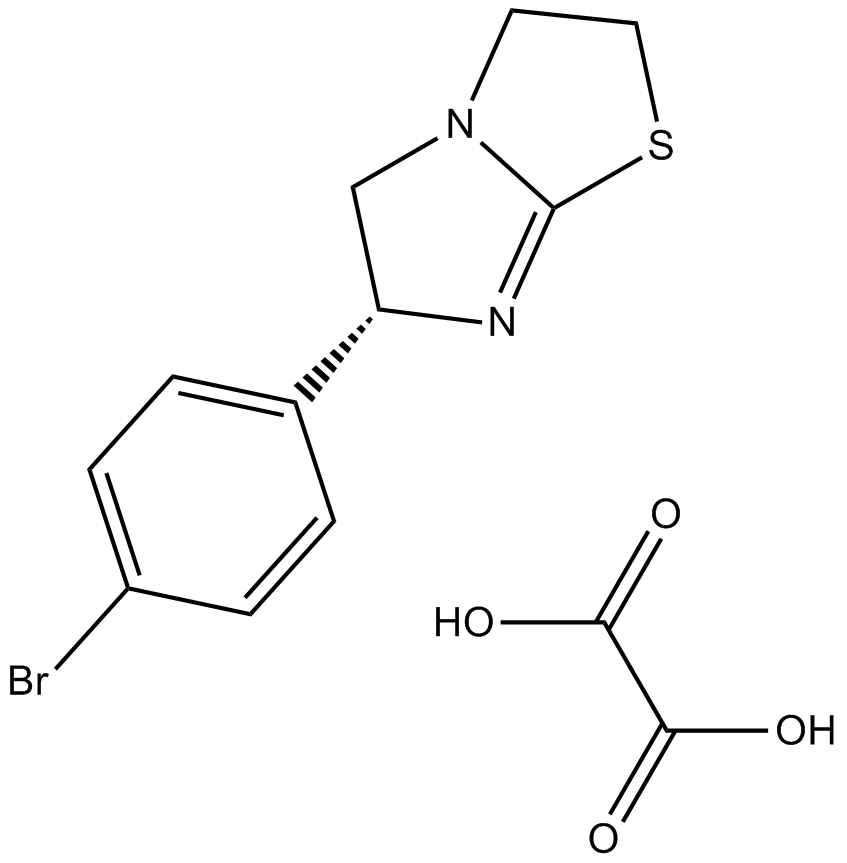
(-)-p-Bromotetramisole Oxalate, a synthetic compound, is mainly being studied for its immunomodulatory qualities and acts as a powerful and random alkaline phosphate inhibitor. It significantly stimulates the chloride channels of the cystic fibrosis transmembrane conductance regulator (CFTR). It is also referred to as l-p-bromotetramisole oxalate or p-bromolevamisole has a molecular weight of 373.22 g/mol . Chemical formula is (-)-(S)-6-(4-Bromophenyl)-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole oxalate. The molecular formula of C13H13BrN2O4S or C11H11BrN2S•C2H2O4, is cell-permeable and its solubility in DMSO is more than 18.7 mg/mL. The chiral molecule l-p-Bromotetramisole Oxalate has a complicated molecular arrangement and because of the asymmetrical carbon centre and attached bromine atom, it displays stereoisomerism. The chemical structure of the compound is shown below:
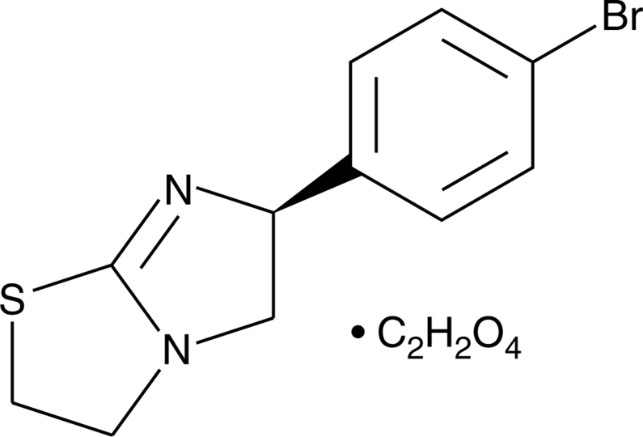
Figure 1: Chemical structure of (-)-p-bromotetramisole oxalate.
The compound is originally derived from another compound called Tetramisole, which is an anthelmintic drug traditionally used to treat animal infections caused by parasitic worms. Tetramisole has various derivatives which play a vital role in the inhibition of the alkaline phosphate activity. alkaline phosphatases are ubiquitous enzymes present in every organ. there are four types of alkaline phosphatases among humans, and each one exhibits a distinct tissue distribution profile. The ease with which their activity can be disclosed has led to a wealth of data indicating associations between changes in activity and diseases.
The chemical is widely used in experimental studies however, there are no clinical uses identified yet. Moreover, there are only fewer but old studies available that have directly employed l-p-bromotetramisole oxalate. explains that phosphate absorption has been linked to enhanced alkaline phosphatase (ALP) activity within the brush border membranes of proximal tubules (BBMV). Levacime-induced ALP suppression had been demonstrated to reduce phosphate absorption in dog and rat brush border membrane vesicles (BBMV) and levamisole administration enhanced phosphate elimination in vivo. However, l-p-bromotetramisole oxalate proved to be more effective than levamisole at inhibiting ALP activity in a variety of tissues, particularly the rat kidney's brush border membrane. Another enzyme group known as protein tyrosine phosphatases is responsible for catalysing protein tyrosine residues' elimination of phosphate groups. These phosphatases are also inhibited by l-p-bromotetramisole oxalate. While higher concentrations of l-p-bromotetramisole oxalate (above 10 mM) significantly reduced the efficiency of crude homogenate acid phosphatase. This implies that the substance targets protein tyrosine phosphatases as well as alkaline phosphatases, indicating a more general inhibitory action on phosphatase enzymes.
To ascertain if l-p-bromotetramisole oxalate's as ALP inhibitors of tetramisole series have the potential to bring back Sarcoma 180/TG's susceptibility to 6-thioguanine, the compound's growth-inhibitory characteristics were assessed in tissue culture. l-p-bromotetramisole oxalate is one of the most powerful potent ALP inhibitors employed for this tumour (IC50 = 1.8x10-6 M), as it is a generally stable, water-soluble variant of this class. Tests conducted in culture revealed that 10-4M of l-p-bromotetramisole was needed to prevent Sarcoma 180/TG proliferation.
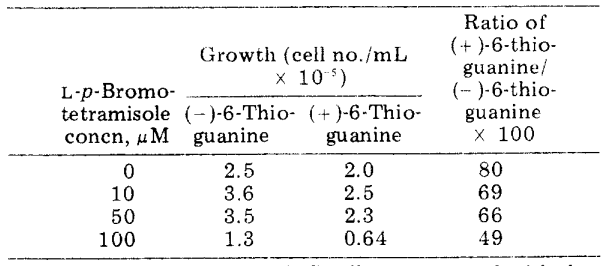
Figure 2: Impact of l-p-bromotetramisole oxalate on the Sarcoma l80/TG cells’ growth in culture.
l-p-bromotetramisole, has been demonstrated as a strong inhibitor of random alkaline phosphatase in the study by Borgers and Thoné . In a variety of rat tissues, complete inhibition was obtained cytochemically at a dose of 0.1 mM; the intestine was unaffected. There was no effect of d-p-bromotetramisole on enzyme activity. Since the inhibitor does not influence the function of particular phosphatases, it is advised to use this medication alongside a specialised phosphatase medium to produce the desired activity exclusively. Thus, l-p-bromotetramisole oxalate is described as a substance that potentially suppresses acid phosphatase enzyme activity in homogenates of rat secretory enamel organs.
In another study by Zaccone, Fasulo and Licata, it was demonstrated that epidermal ionocytes of the Teleost Blennius sanguinolentus were subjected to both light and electron microscopy procedures to determine the location of alkaline phosphatase along with K-dependent nitrophenyl phosphatase. Labyrinth tubules' cytoplasmic surface, apical vesicles and at closer association of plasmic membranes displayed a greater accumulation of the reaction products produced with the various media. However, the incorporation of particular inhibitors, l-p-bromotetramisole oxalate ( at 0.5-1 mM concentration) as well as ouabain, to complete and control media-affected intracellular activity along with that of plasma membranes. Thus the control studies did, in fact, show that in certain specimens cultured with a concentration of 0.5–1 mM of l-p-bromotetramisole oxalate, there was a noticeable decrease in deposits connected to labyrinth tubules, enabling the identification of alkaline phosphatase locations.neuronal processes depend on protein phosphorylation, such as ion channels, exocytosis proteins, neurotransmitter receptors, and enzymes involved in neurotransmitter production. In neuronal cells, tyrosine residues are phosphorylated along with threonine and serine residues. Tyrosine kinases and protein-tyrosine phosphatases both play a vital role in tyrosine residues' degree of phosphorylation within proteins. A buildup of tyrosine-phosphorylated proteins and the stimulation of kinases which are typically kept inactive via dephosphorylation are the results of Vanadate, a well-known inhibitor of enzymes protein-tyrosine phosphatases. The study mainly examined the effects of another tyrosine phosphatase inhibitor that is sodium orthovanadate (Na3VO4) on [Ca2+]i and the release of [3H]NA in PC12 cells. The results imply that, in rat PC12 cells, protein-tyrosine phosphorylation amplifies the Ca2+-induced release of norepinephrine (NA) downstream to [Ca2+] mobilisation. However, the action of l-p-bromotetramisole oxalate was also explained by examining its impact on the excretion of NA in PC12 cells in response to ionomycin. Since there were different inhibitors employed to examine their impact on the ionomycin-stimulated NA release in addition to sodium orthovanadate (Na3VO4), the introduction of l-p-bromotetramisole oxalate greatly increased PC12 cells' 5 µM ionomycin-stimulated release of [3H]NA.
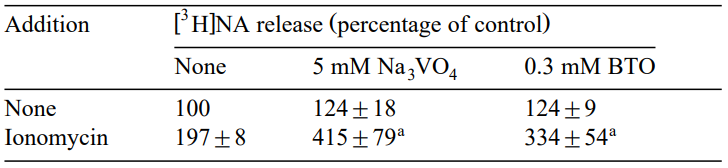
Figure 3: Augmentation of the ionomycin-activated release of [3H]NA via l-p-bromotetramisole oxalate; ap<0.01= significantly distinct value from that observed in the absence of an inhibitor of tyrosine phosphatase.
It was observed that alone l-p-bromotetramisole oxalate had slightly stimulated the Ca2+-induced NA release, at a concentration of 0.3 mM but it did not have any significant impact. Since there exist more than 40 protein-tyrosine phosphatases, it is expected that the inhibitory ranges of Na3VO4 and L-p-bromotetramisole oxalate, in PC12 cells, vary for these phosphatases.













Kommentare