ERK-IN-1 |
| カタログ番号GC33206 |
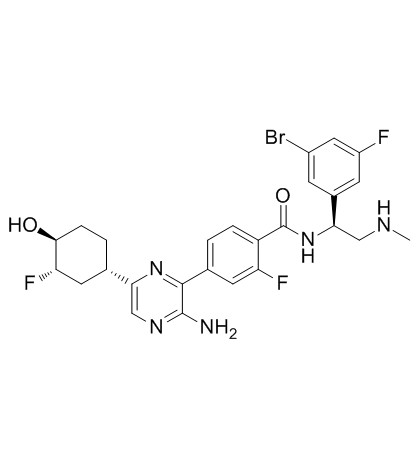
ERK-IN-1 (化合物 B) は、MAPK 経路の変異を活性化することを特徴とする増殖性疾患の治療における経口投与可能な ERK1 および ERK2 阻害剤です。この活性は、特に KRAS 変異 NSCLC、BRAF 変異 NSCLC、KRAS 変異膵臓がん、KRAS 変異結腸直腸がん (CRC)、および KRAS 変異卵巣がんの治療に関連しています。 ERK-IN-1 塩酸塩は、RAF を阻害することもできます。
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1715025-32-3
Sample solution is provided at 25 µL, 10mM.
ERK-IN-1 (compound B) is a RAF and ERK1/2 inhibitor in the treatment of a proliferative disease characterized by activating mutations in the MAPK pathway. The activity is particularly related to the treatment of KRAS-mutant NSCLC (non-small celllung cancer), BRAF-mutant NSCLC, KRAS-mutant pancreatic cancer, KRAS-mutant colorectal cancer (CRC) and KRAS-mutant ovanan cancer[1].
ERK-IN-1 (compound B) (50, 75 mg/kg, p.o., qd/q2d, 27 days) treatment significantly reduces the tumor volume in the Calu-6 human NSCLC subcutaneous tumor xenograft model in mice[1].|| Animal Model:|Calu-6 NSCLC xenograft tumor models in mice[1].|Dosage:|50, 75 mg/kg.|Administration:|Orally either daily (qd) or every other day (q2d) for 27 days.|Result:|Significantly reduced the tumor volume.
[1]. WO2018051306A1.
Average Rating: 5 (Based on Reviews and 13 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















