Eltanexor (KPT-8602) |
| Catalog No.GC19466 |
A second-generation exportin-1 inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
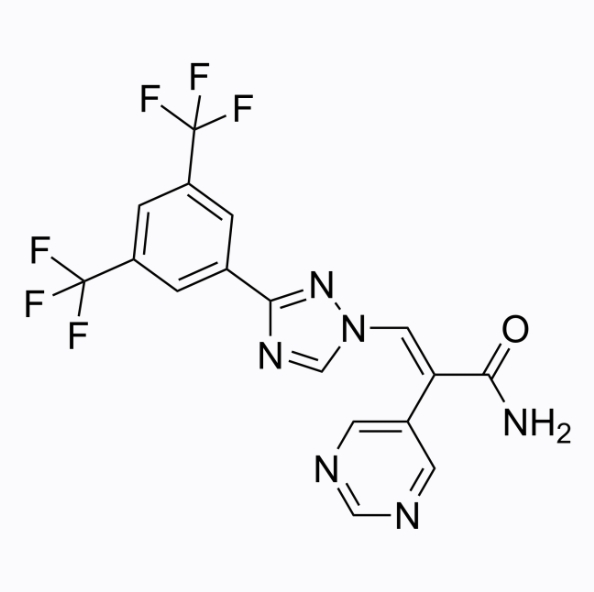
Cas No.: 1642300-52-4
Sample solution is provided at 25 µL, 10mM.
Eltanexor, also known as KPT-8602, is a second-generation exportin-1 inhibitor. KPT-8602 demonstrates potent activity against acute lymphoblastic leukemia. KPT-8602 is well tolerated and highly active against AML blasts and leukemia-initiating cells. Eltanexor shows improved efficacy and in vivo tolerability in hematological malignancies.
KPT-8602 is a potent inhibitor of AML cells in cell-based viability assays[1]. KPT-8602 inhibits XPO1/cargo interactions and nuclear export, induces apoptosis of primary CLL cells and significantly inhibits proliferation of diffuse large B-cell lymphoma cell lines[2]
KPT-8602 is orally bioavailable and has similar pharmacokinetic properties to selinexor, but has markedly reduced (approximately 30-fold less) penetration across the blood−brain barrier. Toxicology studies in rats and monkeys indicate that KPT-8602 has a substantially better tolerability profile, probably due to its inability to penetrate into the CNS, with reduced anorexia, malaise and weight loss compared to selinexor.
KPT-8602 exhibits superior anti-leukemic activity and better tolerability in the AML PDX models tested, with nearly complete elimination of human AML cells in the AML-CN model. KPT-8602 is minimally toxic to normal hematopoietic stem and progenitor cells[1]. KPT-8602 does not accumulate in plasma after repetitive dosing and prolongs survival in a human leukemia xenograft model of AML[2].
Reference:
[1] Etchin J, et al. Leukemia. 2017, 31(1):143-150.
[2] Hing ZA, et al. Leukemia. 2016, 30(12):2364-2372.
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















