Etofenamate |
| Catalog No.GC16705 |
An NSAID
Products are for research use only. Not for human use. We do not sell to patients.
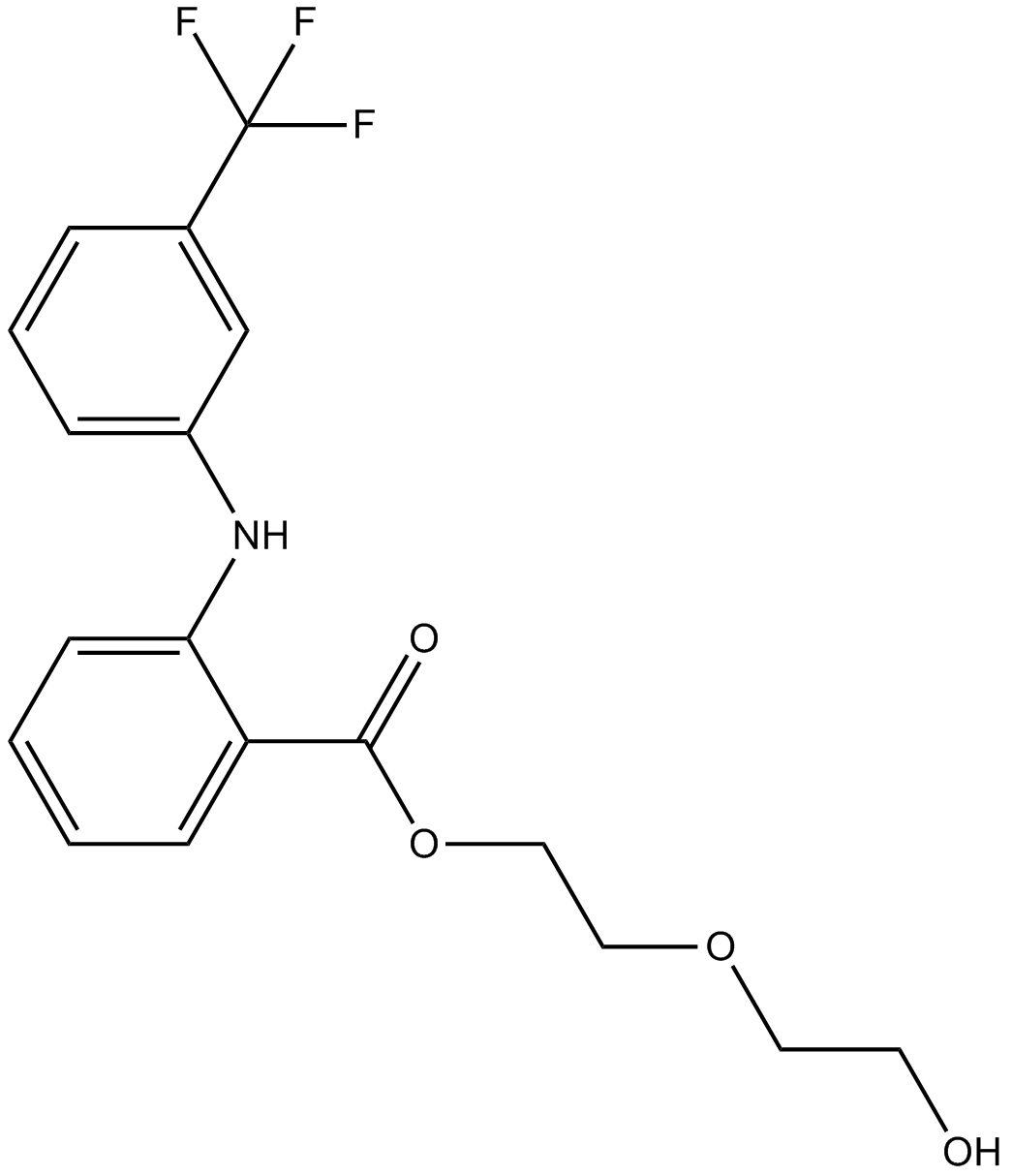
Cas No.: 30544-47-9
Sample solution is provided at 25 µL, 10mM.
Etofenamate is a non-steroidal anti-inflammatory drug used for the treatment joint and muscular pain.
References:
[1]. Bender T, Bariska J, Rojkovich B, Bálint G. Etofenamate levels in human serum and synovial fluid following iontophoresis. Arzneimittelforschung. 2001;51(6):489-92.
[2]. Fraga A, de Almeida M, Moreira-da-Silva V et al. Intramuscular Etofenamate versus Diclofenac in the Relief of Renal Colic : A Randomised, Single-Blind, Comparative Study. Clin Drug Investig. 2003;23(11):701-6.
[3]. Guevara-López U, Uscanga-Sánchez S, Márquez J et al. [Comparative clinical multicenter study to evaluate analgesic effectiveness of intramuscular etofenamate and diclofenac in patients with post-surgical pain]. Cir Cir. 2004 Nov-Dec;72(6):483-90.
[4]. Patiñio JL, et al. [Etofenamate and the analgesic effect in the management of acute pain from spine in the emergency room]. Acta Ortop Mex. 2007 Sep-Oct;21(5):253-5.
[5]. Golcuk Y, Oray D, Atilla OD, Tefennioglu N. Etofenamate associated with Lyell syndrome: a case report. Clin Toxicol (Phila). 2010 Jun;48(5):471-2.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















