6-diazo-5-oxo-L-nor-Leucine (Synonyms: DON, NSC 7365) |
| Catalog No.GC41224 |
6-Diazo-5-oxo-L-nor-Leucine (DON) is a glutamine analog that inhibits glutaminases, a selective, mechanism-based inactivator of glutamine-using enzymes.
Products are for research use only. Not for human use. We do not sell to patients.
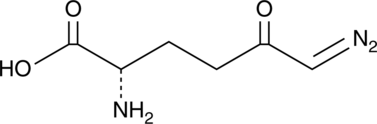
Cas No.: 157-03-9
Sample solution is provided at 25 µL, 10mM.
6-Diazo-5-oxo-L-nor-Leucine (DON) is a glutamine analog that inhibits glutaminases, a selective, mechanism-based inactivator of glutamine-using enzymes[1-3].
6-Diazo-5-oxo-L-nor-Leucine(10-2µM-102µM ;48h) inhibited cell proliferation, and the inhibitory effect increased with the increase of concentration[4]. 6-Diazo-5-oxo-L-nor-Leucine (0.3 mM; 1 h) inhibited glutamine catabolism in WI-L2 cells[1].Treatment with 6-Diazo-5-oxo-L-nor-Leucine at 50 µM decreased colony formation in S2VP10 cells[5].
6-Diazo-5-oxo-L-nor-Leucine(1 mg/kg;5 days a week)decreased tumor progression as well as end-of-study tumor weight and volume[6].6-Diazo-5-oxo-l-norleucine as a Glutaminase(GLS) inhibitor that produces long lasting pain relief when applied to the inflamed paw of arthritic rats, DOX(2mM, 0.05ml;2times) can reduce the increase of the skin for vesicular transporters (VGluT2), GLS and glutamate immunoreactivity (IR) caused by surgery in a post-incisional model[7,8].
References:
[1]. Willis RC, Seegmiller JE. The inhibition by 6-diazo-5-oxo-l-norleucine of glutamine catabolism of the cultured human lymphoblast. J Cell Physiol. 1977 Dec;93(3):375-82. doi: 10.1002/jcp.1040930308. PMID: 22551.
[2]. Thangavelu K, Pan CQ, et,al. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc Natl Acad Sci U S A. 2012 May 15;109(20):7705-10. doi: 10.1073/pnas.1116573109. Epub 2012 Apr 26. PMID: 22538822; PMCID: PMC3356676.
[3]. Rais R, Lemberg KM, et,al. Discovery of DRP-104, a tumor-targeted metabolic inhibitor prodrug. Sci Adv. 2022 Nov 18;8(46):eabq5925. doi: 10.1126/sciadv.abq5925. Epub 2022 Nov 16. PMID: 36383674; PMCID: PMC9668306.
[4]. Leone RD, Zhao L, et,al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019 Nov 22;366(6468):1013-1021. doi: 10.1126/science.aav2588. Epub 2019 Nov 7. PMID: 31699883; PMCID: PMC7023461.
[5]. Sharma NS, Gupta VK, et,al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J Clin Invest. 2020 Jan 2;130(1):451-465. doi: 10.1172/JCI127515. PMID: 31613799; PMCID: PMC6934212.
[6]. Sharma NS, Gupta VK, et,al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J Clin Invest. 2020 Jan 2;130(1):451-465. doi: 10.1172/JCI127515. PMID: 31613799; PMCID: PMC6934212.
[7]. Crosby HA, Miller KE. Evaluating the Analgesic Effect of the GLS Inhibitor 6-Diazo-5-Oxo-L-Norleucine in Vivo. Pharm Pharmacol Int J. 2016;3(3):00055. doi: 10.15406/ppij.2015.03.00055. Epub 2016 Jan 8. PMID: 29888760; PMCID: PMC5993434.
[8]. Miller KE, Hoffman EM, et,al. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol Ther. 2011 Jun;130(3):283-309. doi: 10.1016/j.pharmthera.2011.01.005. Epub 2011 Jan 26. PMID: 21276816; PMCID: PMC5937940.
Average Rating: 5 (Based on Reviews and 20 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *





