Alamethicin F50 (Synonyms: 18-L-Glutamine Alamethicin I) |
| Catalog No.GC42763 |
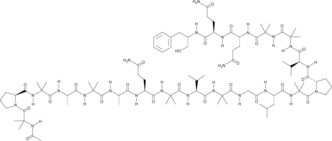
Alamethicin F50 is a peptaibol isolated from Trichoderma.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 56165-93-6
Sample solution is provided at 25 µL, 10mM.
Alamethicin F50 is a peptaibol isolated from Trichoderma. It is a polymer of 20 amino acids that includes modified and non-proteinogenic amino acids. Alamethicin F50 is a mixture of 13 different peptides, with the most abundant designated as alamethicin F50/5. Alamethicin F50 forms voltage-dependent ion channels in lipid membranes and acts as a lytic agent. It induces lysis of leukocytes (IC50 = 54 and 80 μM for rat mast cells and mouse spleen lymphocytes, respectively) and exhibits antibacterial activity against a panel of 8 mollicutes (MICs = 1.56-12.5 μM).
Average Rating: 5 (Based on Reviews and 14 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















