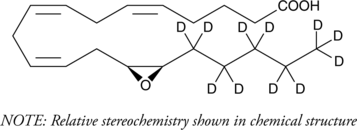
(±)14(15)-EET-d11 (Synonyms: (±)14,15-EET-d11, (±)14,15-EpETrE-d11) |
| Catalog No.GC46259 |
A neuropeptide with diverse biological activities
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 2699608-29-0
Sample solution is provided at 25 µL, 10mM.
(±)14(15)-
1.Chacos, N., Falck, J.R., Wixtrom, C., et al.Novel epoxides formed during the liver cytochrome P-450 oxidation of arachidonic acidBiochem. Biophys. Res. Commun.104(3)916-922(1982) 2.Oliw, E.H., Guengerich, F.P., and Oates, J.A.Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediatesJ. Biol. Chem.257(7)3771-3781(1982) 3.Jiang, J.-X., Zhang, S.-J., Xiong, Y.-K., et al.EETs attenuate ox-LDL-induced LTB4 production and activity by inhibiting p38 MAPK phosphorylation and 5-LO/BLT1 receptor expression in rat pulmonary arterial endothelial cellsPLoS One10(6)e0128278(2015) 4.Oltman, C.L., Weintraub, N.L., VanRollins, M., et al.Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculationCirc. Res.83(9)932-939(1998) 5.Nithipatikom, K., Moore, J.M., Isbell, M.A., et al.Epoxyeicosatrienoic acids in cardioprotection: Ischemic versus reperfusion injuryAm. J. Physiol. Heart Circ. Physiol.291(2)H537-H542(2006)
Average Rating: 5 (Based on Reviews and 14 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















